Conditional Dystrophin Ablation: A New Window into Muscle Development and Brain Function
Dystrophin: A Critical Protein for Muscle Health
Dystrophin is a key structural protein that stabilizes muscle fibers and connects them to the surrounding extracellular matrix. A loss or mutation in the dystrophin gene causes severe muscle-wasting disorders such as Duchenne Muscular Dystrophy (DMD). DMD is a progressive and fatal genetic disease that leads to muscle weakness, loss of mobility, and early death. While many studies have focused on models where dystrophin is completely absent from all tissues, the specific effects of targeted dystrophin deletion—especially in skeletal muscle stem cells—have remained largely unexplored.
What Did This Study Aim to Uncover?
In this groundbreaking preprint, Dr. Matthew Alexander, Dr.Karyn Esser, Dr. Louis Kunkel, and their team investigated what happens when dystrophin is selectively removed from muscle satellite cells—the resident stem cells responsible for muscle repair and regeneration. Their goal was to understand how dystrophin affects early muscle development, satellite cell fate decisions, extracellular matrix (ECM) regulation, and even behavioral and neurocognitive functions.
Conditional Deletion of Dystrophin in Muscle Stem Cells
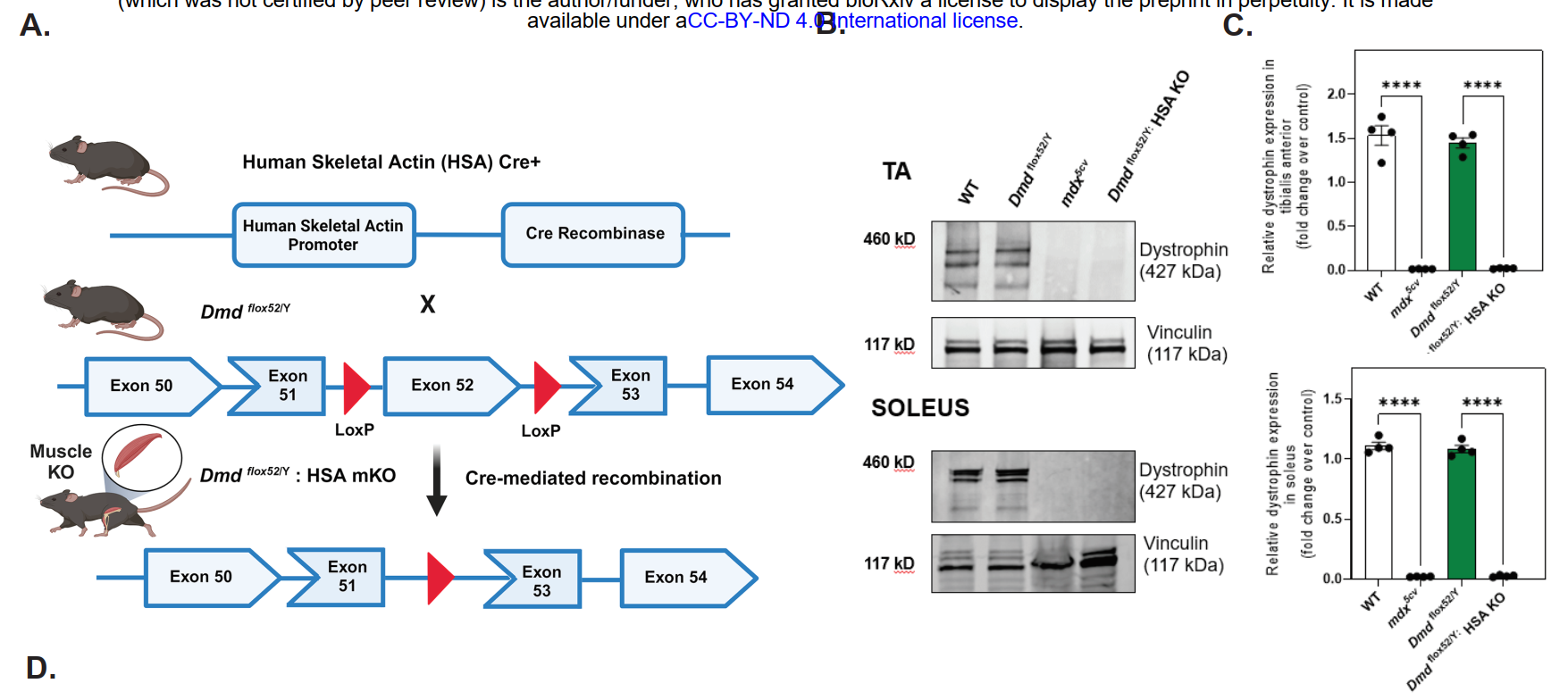
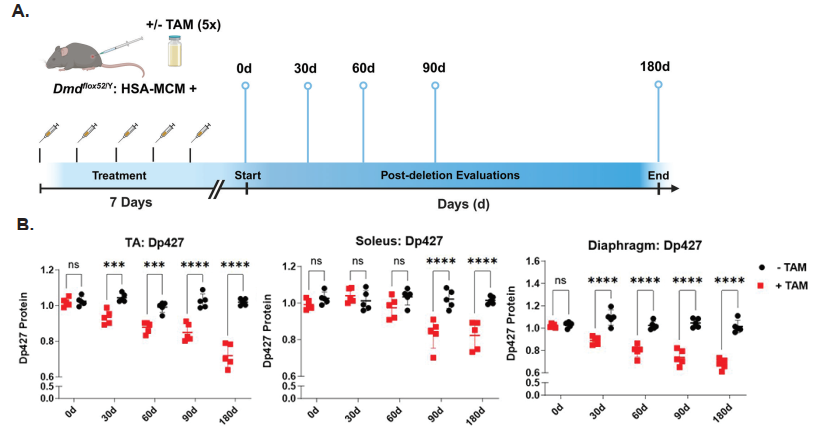
To precisely remove dystrophin from satellite cells, the researchers used the Cre-LoxP system. They engineered mice carrying floxed alleles of the dystrophin gene (Dmd^flox/flox) and crossed them with Pax7-Cre mice, in which the enzyme Cre recombinase is expressed specifically in satellite cells. The result was a satellite cell-specific dystrophin knockout, referred to as scKO mice.
Impaired Muscle Growth in the Absence of Satellite Cell Dystrophin
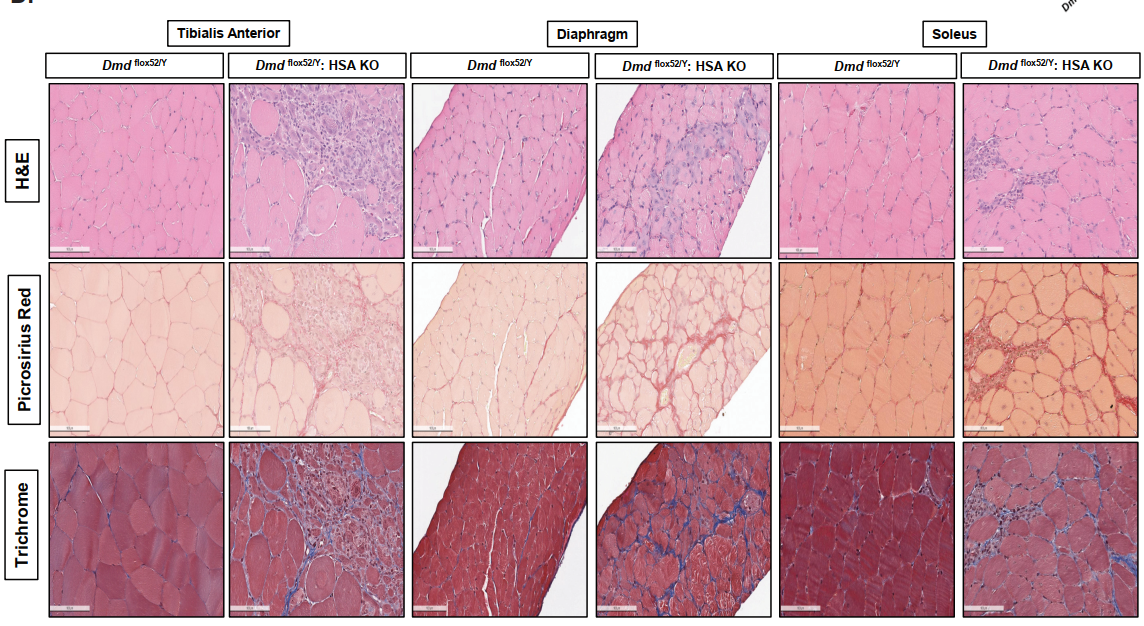
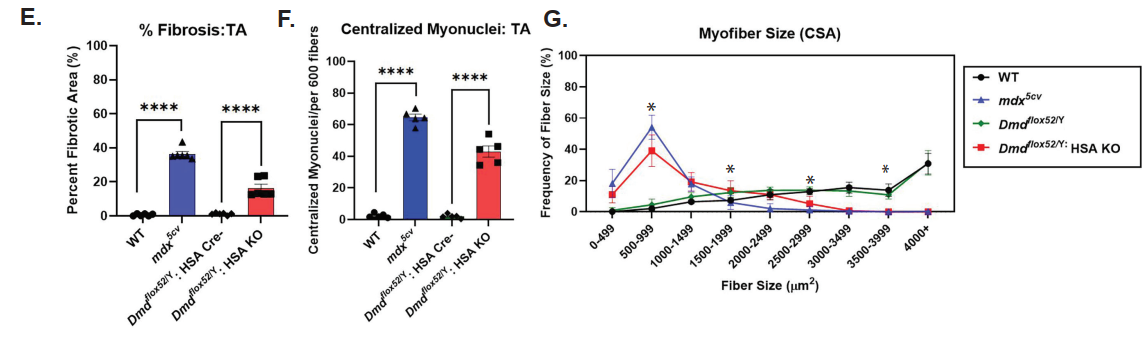
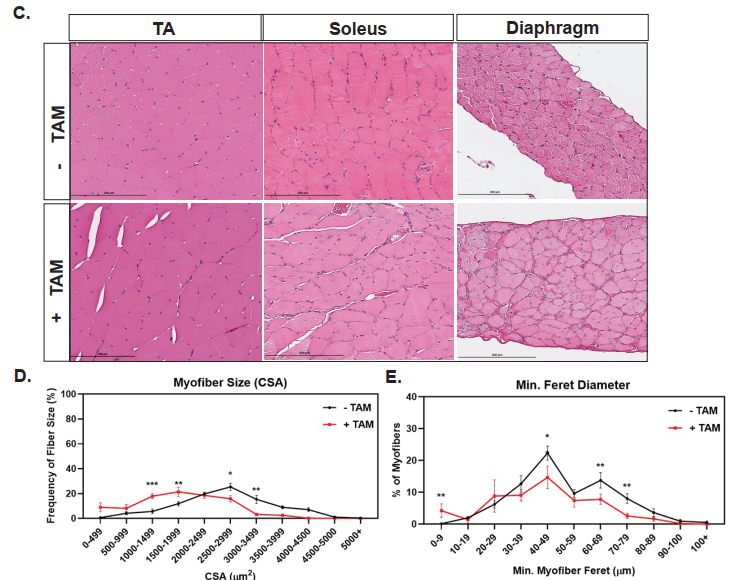
One of the central findings was that scKO mice showed severe structural and regenerative defects in skeletal muscles. Histological analysis revealed smaller muscle fibers, increased fibrosis, and a higher number of centrally located nuclei—hallmarks of ongoing muscle regeneration and damage. The regenerative capacity of these muscles was significantly reduced following injury, highlighting that dystrophin is essential not only for adult muscle maintenance but also during the early phases of muscle formation.
Disruption of Satellite Cell Fate Determination
Single-cell RNA sequencing and gene expression profiling revealed that satellite cells lacking dystrophin had disrupted differentiation trajectories. Instead of progressing smoothly through myogenic lineage steps—from activated myoblasts to myocytes and mature fibers—these cells displayed defective transitions and abnormal gene expression profiles. This suggests that dystrophin helps guide satellite cells through proper developmental programs during muscle regeneration.
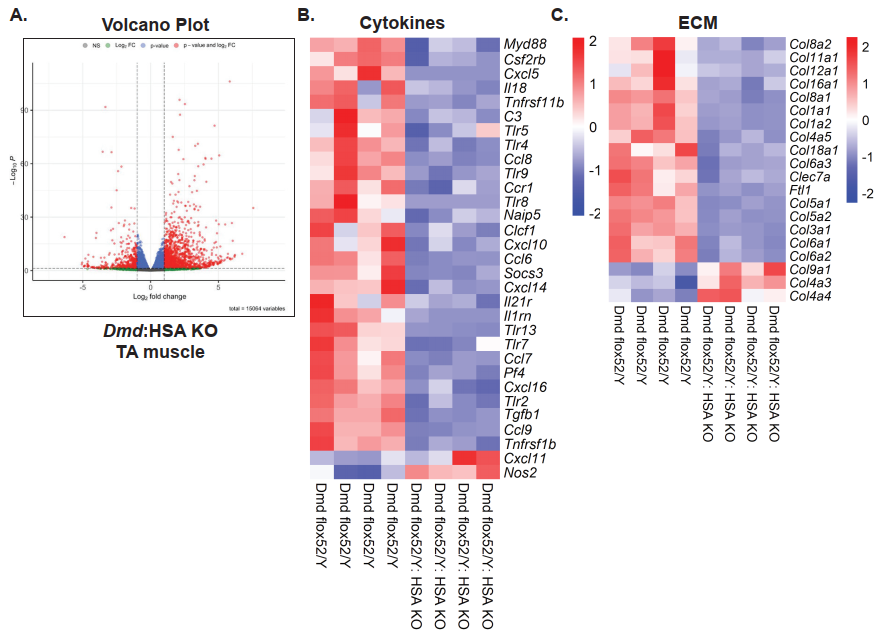
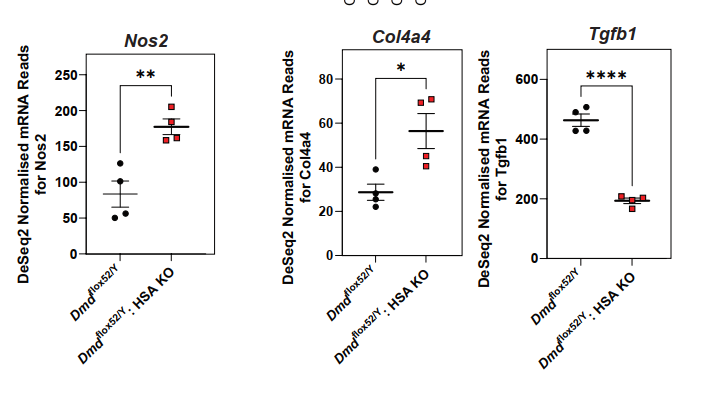
A Surge in Extracellular Matrix Remodeling
The authors observed a striking upregulation of extracellular matrix (ECM)-related genes, such as collagens (Col1a1, Col3a1), proteoglycans, and matrix-modifying enzymes (e.g., MMP2), in scKO muscles. These changes suggest an ECM remodeling response that may reflect or exacerbate tissue fibrosis and architectural disorganization. This expands dystrophin’s role from being a purely structural membrane protein to a regulator of ECM homeostasis during muscle repair.
Neurological Consequences: Cognitive and Behavioral Impairments
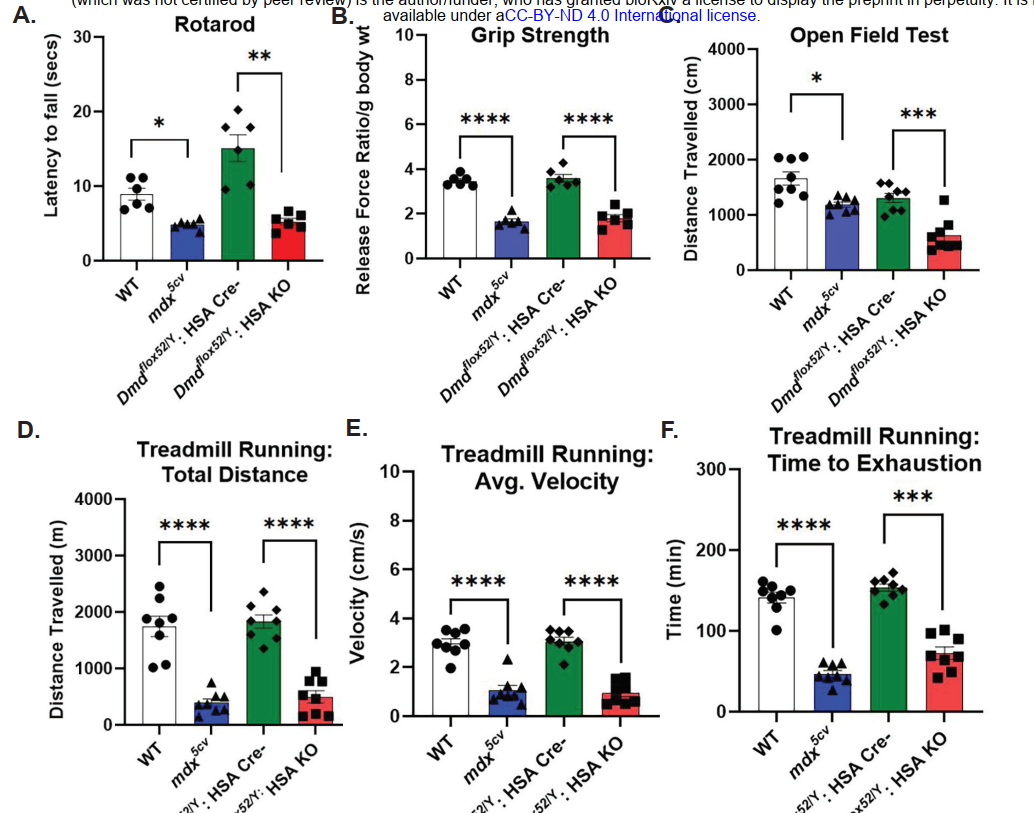
One of the key highlights of this study was the investigation of tissue-specific dystrophin deletion and its impact on behavior. In the scKO (or Dmd mKO) model, where dystrophin was selectively ablated in skeletal muscle, behavioral assessments revealed reduced physical activity, increased time spent in dark corners, and lower exploratory behavior. Although this model was designed to study the muscular consequences of dystrophin loss, the observed behavioral alterations suggest a possible functional interaction between muscle and brain—potentially mediated through muscle-to-brain signaling pathways or developmental muscle–neural interactions during embryogenesis.
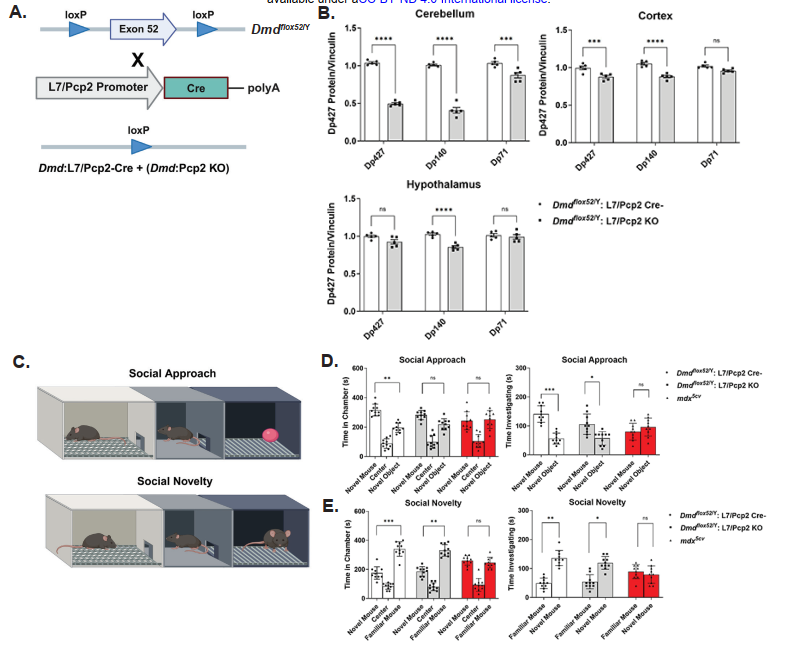
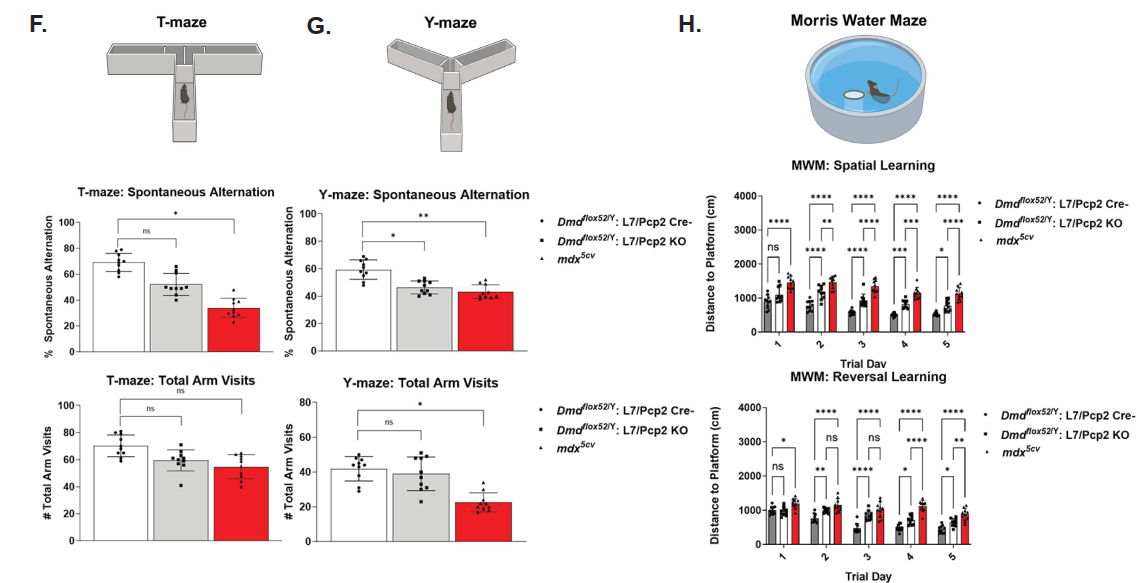
In contrast, the Dmd:Pcp2 KO model, which specifically deletes dystrophin in Purkinje neurons of the cerebellum, exhibited clear neurobehavioral deficits including impaired working memory, spatial cognition, social interaction, and social memory. These findings underscore the essential role of the brain-specific dystrophin isoform (Dp427c) in regulating cognitive and behavioral functions. Altogether, the study demonstrates that dystrophin is not only critical for maintaining muscle integrity and function, but also plays indispensable roles in proper neurobehavioral performance.
Anxiety and Motor Behavior Alterations
In addition to cognitive tests, scKO mice also displayed altered motor activity and anxiety-like behavior. They were less active, spent more time in the periphery of open fields, and exhibited less exploratory behavior. These phenotypes further support a systemic effect of muscle dysfunction on central nervous system pathways.
Molecular Insights Confirm Phenotypic Changes
Bulk and single-cell RNA-seq analyses provided deeper insight into the molecular landscape altered by dystrophin deletion. Key affected pathways included:
- Myogenic differentiation (Myod1, Myog downregulation)
- ECM remodeling (upregulation of Col1a1, Col3a1, MMP2)
- Immune signaling (TGF-β signaling, interferon responses)
- Cell adhesion and migration
- Neurodevelopmental genes related to memory and synaptic plasticity
These molecular alterations were consistent with the observed muscle pathology and behavioral impairments.
Why This Study Is Important
This study is the first to show that dystrophin loss specifically in muscle stem cells leads to widespread and unexpected consequences, not only in muscle development and regeneration, but also in behavior and cognition. It positions dystrophin as a multifaceted regulator—one that shapes not only the muscle structure but also systemic physiology, possibly through muscle-brain signaling axes.
Looking Forward: Open Questions and Future Directions
This research opens the door to many important questions:
- Does dystrophin deletion at different developmental stages (e.g., embryonic vs. adult) lead to distinct outcomes?
- What are the precise mechanisms through which muscle dysfunction impacts the brain?
- Could targeting ECM remodeling pathways help mitigate muscle or cognitive symptoms?
- Does dystrophin have a similar regulatory role in other tissue-resident stem cells?











| Field | Details |
|---|---|
| Title | Conditional Dystrophin ablation causes profound effects on muscle development, neurobehavior, and extracellular matrix pathways |
| Authors | Muthukumar Karuppasamy, Katherine G. English, James R. Conner, Shelby N. Rorrer, Michael A. Lopez, David K. Crossman, Jodi R. Paul, Miguel A. Monreal-Gutierrez, Karen L. Gamble, Karyn A. Esser, Jeffrey J. Widrick, Louis M. Kunkel, Matthew S. Alexander |
| Corresponding Author | Matthew S. Alexander (matthewalexander@uabmc.edu) |
| Publication Date | February 1, 2025 (Preprint on bioRxiv) |
| Journal | bioRxiv (preprint server for biology) |
| Keywords | Dystrophin, DMD, muscular dystrophy, skeletal muscle, Dystrophinopathy |
| Methods Used | CRISPR/Cas9 gene editing, conditional knockout mouse generation, histology (H&E, trichrome, picrosirius red), immunofluorescence, western blot, muscle function tests, behavioral assays (rotarod, grip strength, Morris water maze), RNA-seq, qPCR |
| Study Type | Preclinical tissue-specific gene function study in conditional mouse models |
| DOI | 10.1101/2025.01.30.635777 |














