A New Horizon in Treating Duchenne Muscular Dystrophy: AAV-Mediated Gene Therapy
Introduction
Duchenne muscular dystrophy (DMD) is one of the most severe genetic muscle disorders, caused by mutations in the dystrophin gene—one of the largest known human genes (over 2.2 megabases). Since the discovery of the dystrophin gene, considerable efforts have been made to develop gene therapy solutions, particularly using adeno-associated virus (AAV) vectors. This article presents a comprehensive yet concise summary of the latest advancements in AAV-based gene therapy for DMD.
Major Challenges in Gene Therapy for DMD
- Large Gene Size: The full-length dystrophin gene is too large to be packaged in AAV vectors.
- Widespread Muscle Involvement: Effective therapy requires gene delivery to over 650 skeletal muscles and cardiac muscle.
- Immune Response: Immunogenicity against the vector or dystrophin protein can hinder therapeutic efficacy.
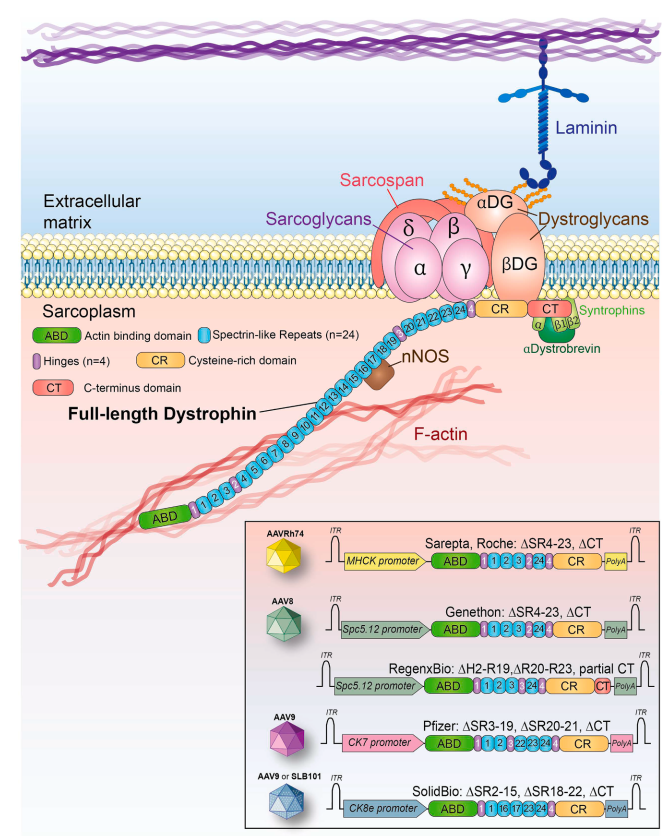
Truncated Dystrophin Constructs: A Viable Alternative
Clinical observations from patients with milder Becker muscular dystrophy (BMD) revealed that smaller, truncated forms of dystrophin can retain partial functionality. This insight led to the design of synthetic micro- and mini-dystrophins that are compatible with AAV vector limitations and capable of providing therapeutic benefit.
Clinical Trials: Progress and Limitations
Six biotech companies have conducted or are conducting clinical trials using AAV-μDys vectors. Sarepta Therapeutics’ gene therapy product became the first to receive FDA approval in 2024. While some patients exhibited modest clinical improvements and slower disease progression, robust muscle strength gains have yet to be consistently demonstrated. Moreover, several adverse events—including immune responses, liver toxicity, and rare fatal outcomes—have highlighted the need for further optimization.
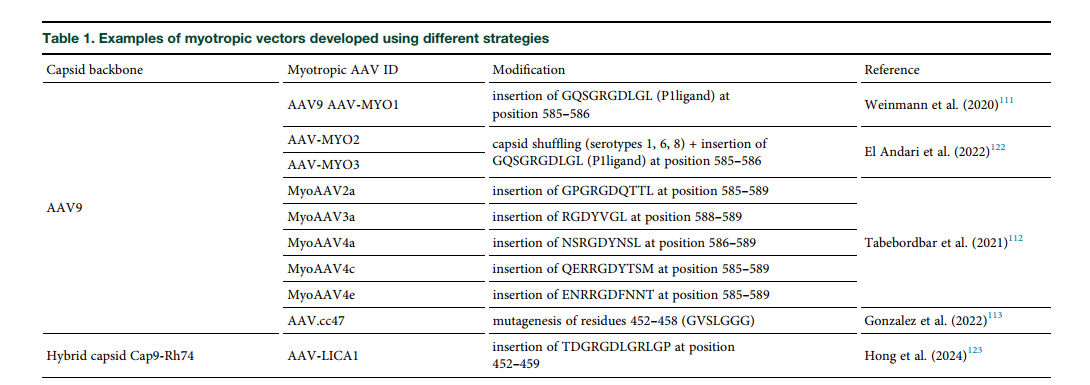
Innovations in AAV Vector Design
Recent advancements focus on developing muscle-specific (myotropic) AAV capsids that efficiently target muscle tissues at lower doses and with reduced liver uptake. Early clinical data from trials using these engineered capsids are promising, showing increased dystrophin expression with fewer adverse effects.
Overcoming AAV Packaging Constraints
To express larger dystrophin constructs, researchers have explored:
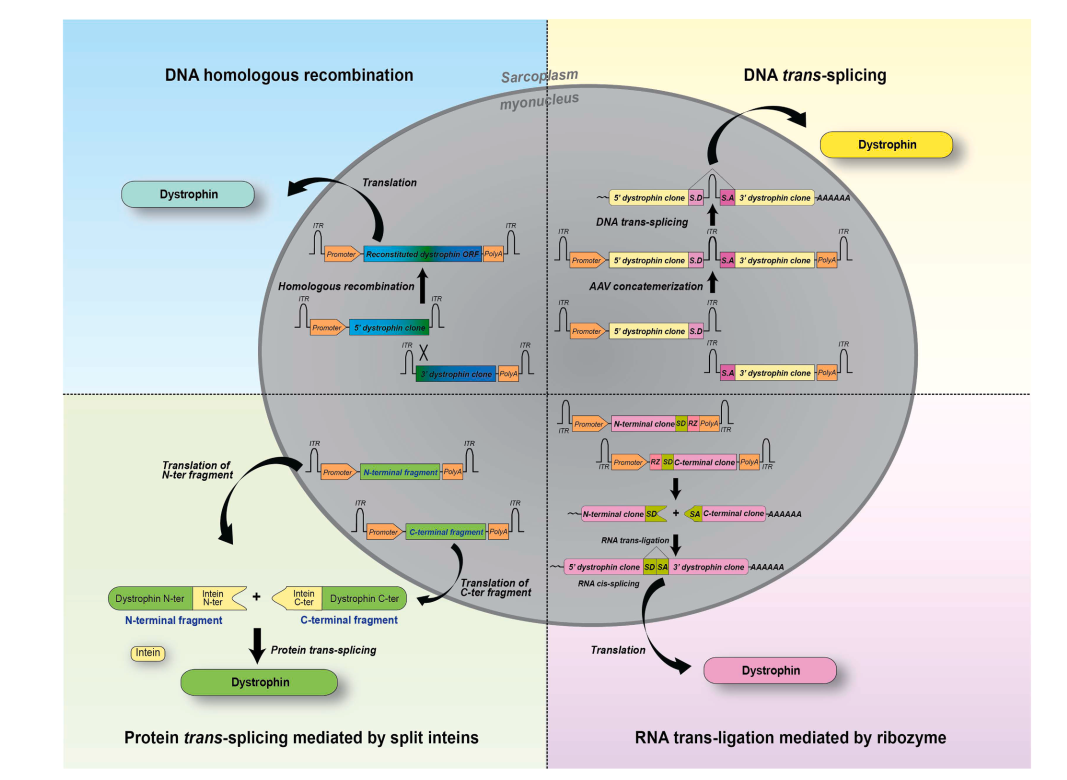
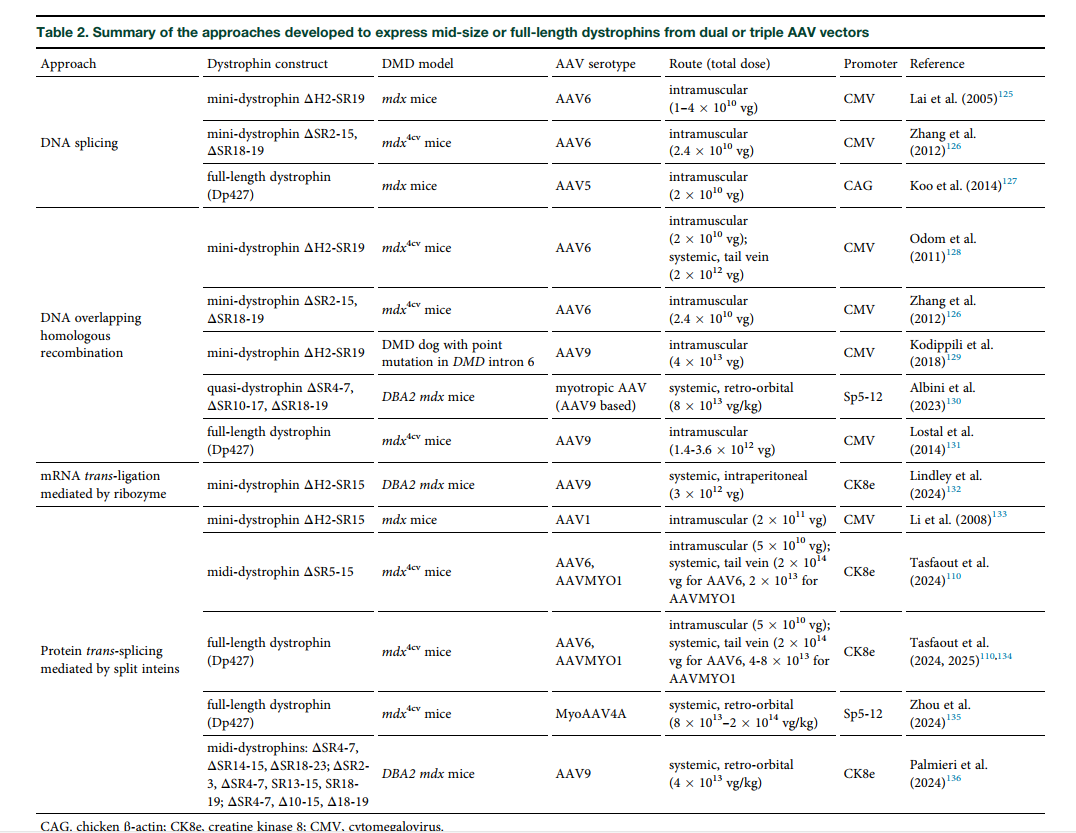
- Dual and triple AAV vectors to reassemble the gene via DNA recombination or trans-splicing.
- Protein trans-splicing using split inteins to join smaller polypeptides into full-length proteins.
- RNA-based strategies, such as ribozyme-mediated mRNA ligation.
Although encouraging in preclinical models, these methods still face significant efficiency challenges in human applications.
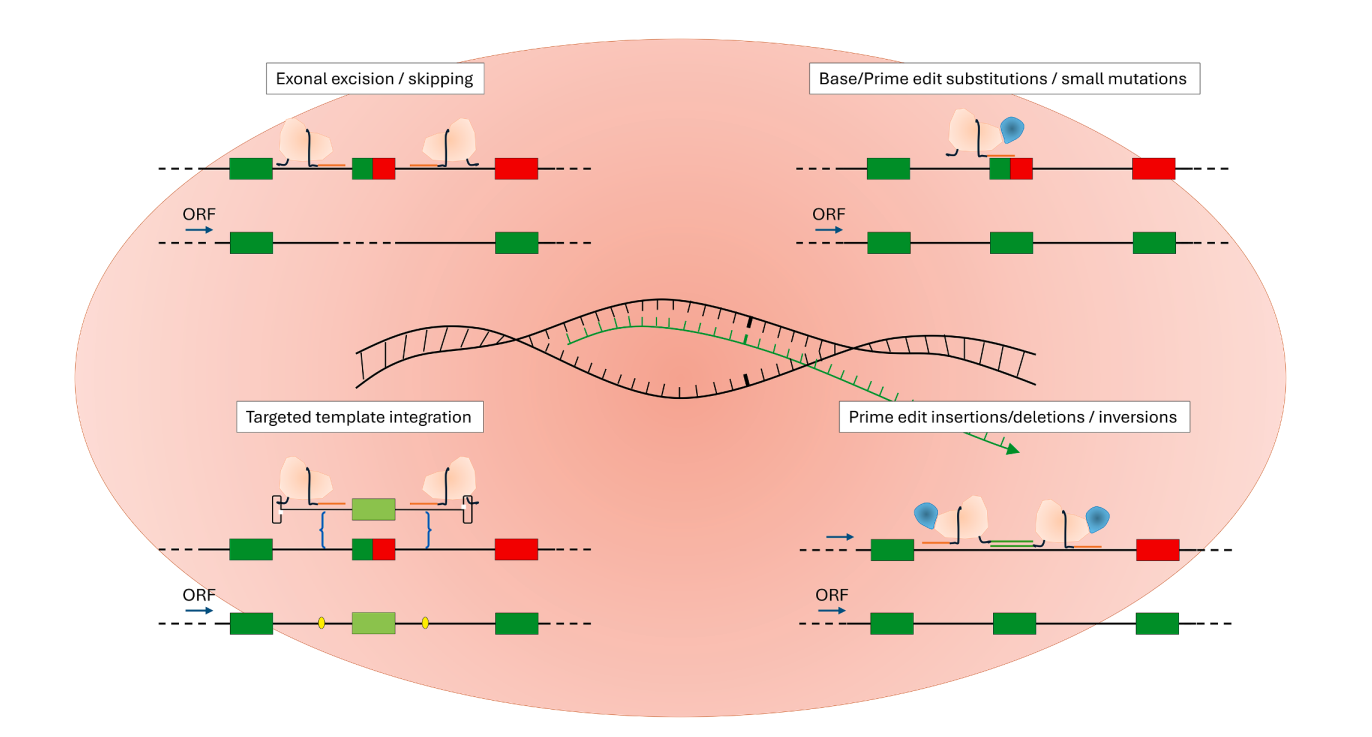
The Promise of Genome Editing
CRISPR-based gene editing strategies are being developed to correct dystrophin mutations directly in patient genomes. These include:
- Exon skipping or excision using CRISPR-Cas9.
- Base and prime editing to correct point mutations precisely.
- Integration of missing sequences through homology-directed repair (HDR) or alternative recombination strategies.
While early animal studies are promising, clinical translation requires further work to ensure safe and efficient delivery, particularly to skeletal and cardiac muscles.
Conclusion
Although the FDA approval of the first gene therapy product for DMD marks a historic milestone, current therapies provide only modest benefit. The field is rapidly evolving, with research underway to enhance vector efficacy, minimize immune responses, and develop more potent genome editing approaches. With continued innovation and support, a future with more effective and lasting treatments for DMD is within reach.



| Field | Details |
|---|---|
| Title | The road toward AAV-mediated gene therapy of Duchenne muscular dystrophy |
| Authors | Niclas E. Bengtsson, Hichem Tasfaout, Jeffrey S. Chamberlain |
| Corresponding Author | Niclas E. Bengtsson (niclasb@uw.edu) Hichem Tasfaout (tasfaout@uw.edu) Jeffrey S. Chamberlain (jsc5@uw.edu) |
| Publication Date | May 2025 |
| Journal | Molecular Therapy, Vol. 33, No. 5 |
| Keywords | Duchenne muscular dystrophy, gene therapy, AAV vectors, micro-dystrophin, CRISPR-Cas9, myotropic AAV, muscle-targeted delivery |
| Methods Used | Review of preclinical and clinical studies including: – AAV vector development – micro/midi/full-length dystrophin delivery – split-intein protein trans-splicing – gene editing (CRISPR, base/prime editing) – dual/triple vector strategies |
| Study Type | Review Article |
| DOI | 10.1016/j.ymthe.2025.03.065 |