Overview
Emery-Dreifuss muscular dystrophy (EDMD) is a rare and genetically complex neuromuscular disorder characterized by progressive muscle wasting, early-onset contractures, and life-threatening cardiac conduction issues. The disorder exhibits significant clinical variability, even among family members with the same genetic mutations, making diagnosis and treatment challenging.
Genetic Complexity and Study Approach
- Genetic Heterogeneity: Only about 50% of EDMD cases have been linked to mutations in six nuclear envelope genes.
- Limitations of Traditional Approaches: Genome-wide association studies (GWAS) have struggled to identify all causative mutations due to the disorder’s complex genetic landscape.
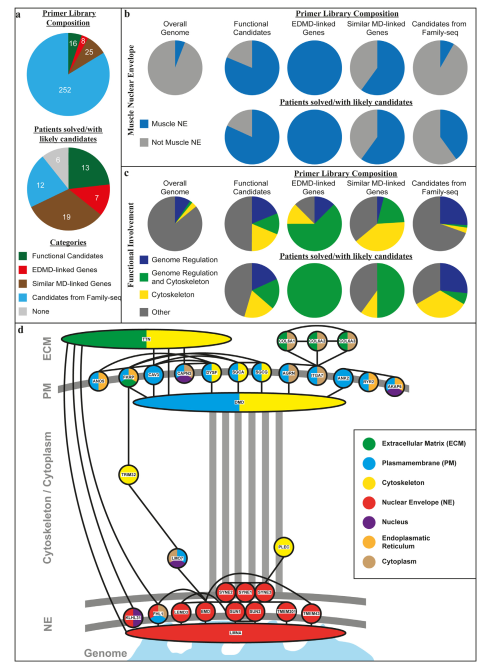
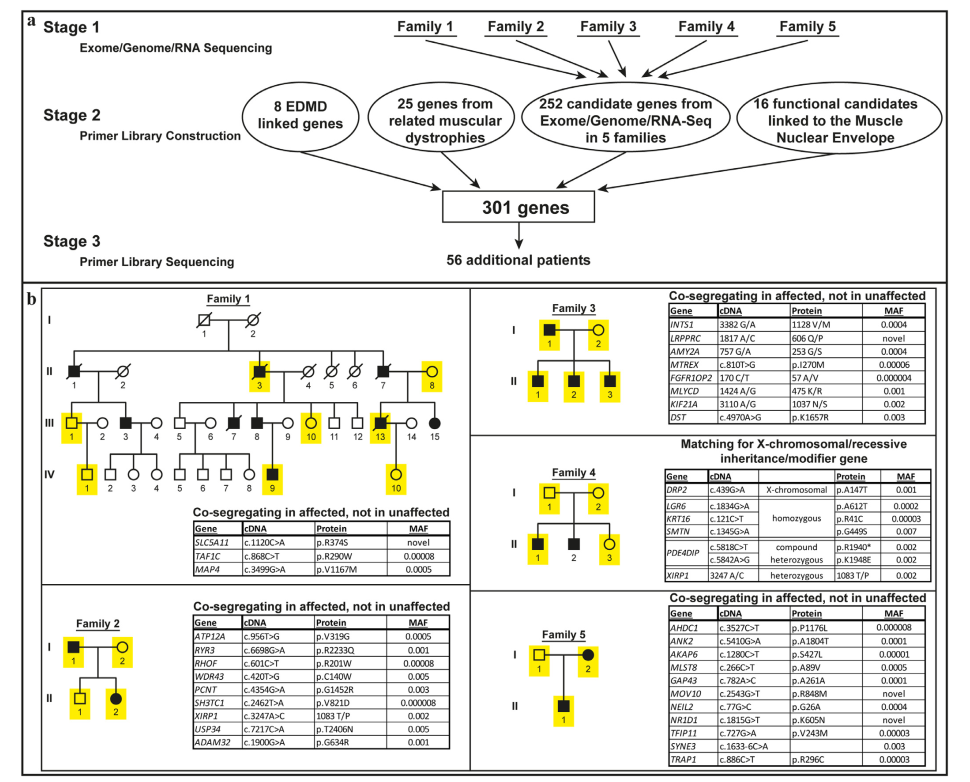
- Multistage Sequencing Strategy: Researchers targeted 301 genes, categorized into four key groups:
- Previously known EDMD-linked genes.
- Genes associated with related muscular dystrophies.
- Candidate genes identified through exome sequencing in five families.
- Functional candidates involved in muscle-specific nuclear processes.
Key Findings
- Identified Mutations: The study applied this strategy to 56 patients with EDMD-like phenotypes, leading to the identification of clear mutations in 21 patients.
- New Candidate Genes: Previously missed mutations in known EDMD genes and novel genes encoding nuclear envelope proteins were identified.
- Role of Gene Regulation: The findings suggest that altered gene regulation, rather than mechanical instability, may be the primary pathomechanism driving EDMD.
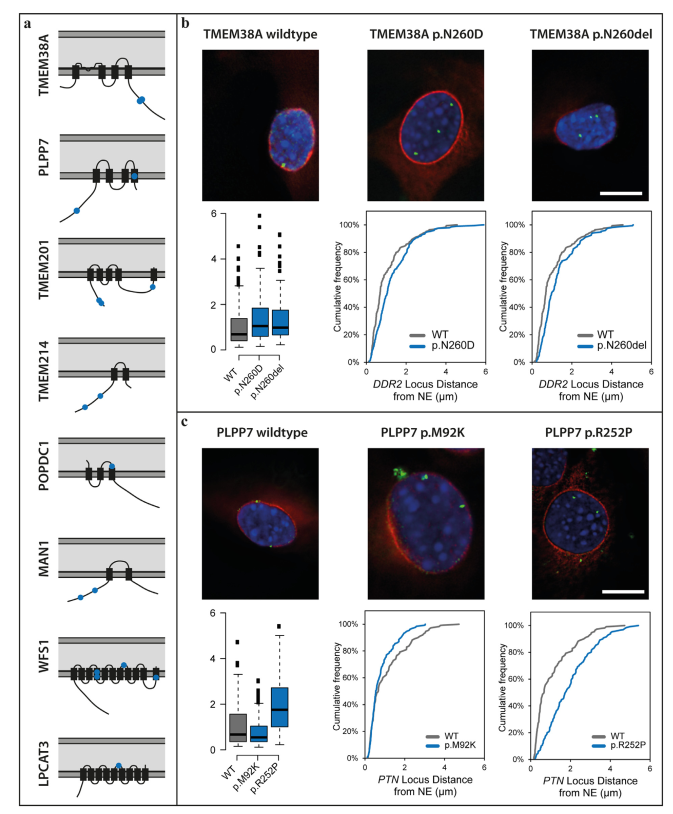
- Implications for Future Research: Disruptions in gene positioning within the nucleus were observed in EDMD patients, offering new insights into disease mechanisms.
Conclusion and Future Implications
This research advances our understanding of Emery-Dreifuss muscular dystrophy (EDMD) by identifying new genetic mutations and reinforcing the importance of gene regulation in its pathogenesis. The study's multistage sequencing strategy provides a promising tool for diagnosing and exploring the genetic basis of EDMD and potentially other complex disorders. These findings open new avenues for research and could lead to more targeted and timely interventions for patients suffering from this debilitating disease.







| Published | 11/26/2019 |
| Address | https://doi.org/10.1016/j.ebiom.2019.11.048 |
| Authors | Peter Meinkea,b, Alastair R.W. Kerra, Rafal Czapiewskia, Jose I. de las Herasa, Charles R. Dixona, Elizabeth Harrisc, Heike Kolbel € d, Francesco Muntonie,f, Ulrike Scharad, Volker Straubc, Benedikt Schoserb, Manfred Wehnertg, Eric C. Schirmer |














