Advances in Oligonucleotide-Based Therapies for Facioscapulohumeral Muscular Dystrophy (FSHD)
1. Introduction to FSHD
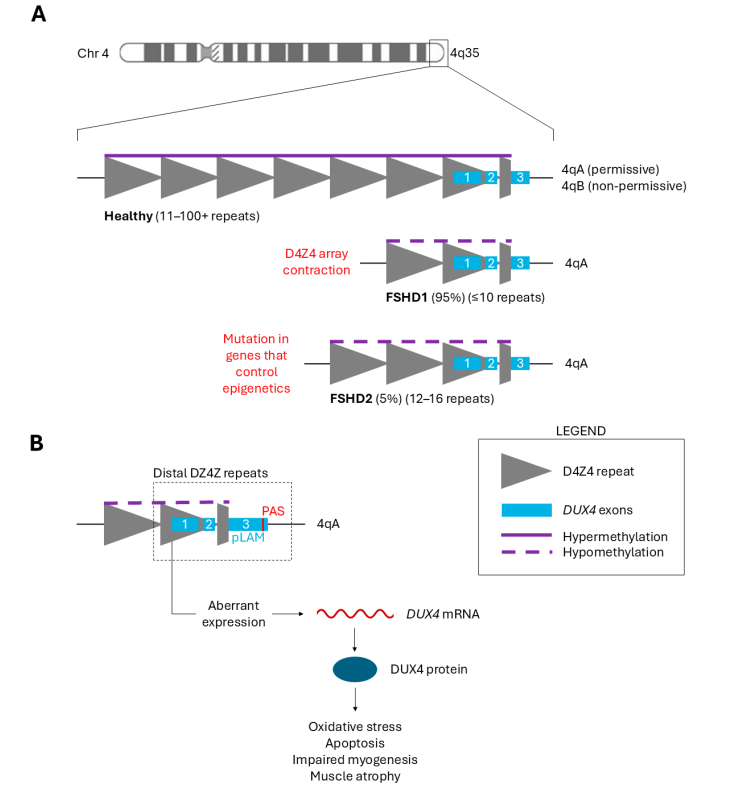
Facioscapulohumeral muscular dystrophy (FSHD) is a progressive genetic disorder primarily affecting facial, shoulder, and upper arm muscles. Over time, the disease can cause severe muscle atrophy. The key molecular feature of FSHD is the aberrant activation of the DUX4 gene, which leads to toxic effects in muscle tissue. The disease is classified into:
- FSHD1: Caused by contraction of the D4Z4 repeat array.
- FSHD2: Caused by mutations in epigenetic regulators, leading to loss of DUX4 repression.
2. Role of DUX4 in FSHD Pathogenesis
Normally, DUX4 is only active during early embryonic development. However, in FSHD patients, DUX4 expression persists in muscle tissue, triggering a cascade of damaging effects, including:
- Muscle cell death due to toxic protein accumulation.
- Inflammatory response leading to further muscle degeneration.
- Mitochondrial dysfunction, impairing muscle function.
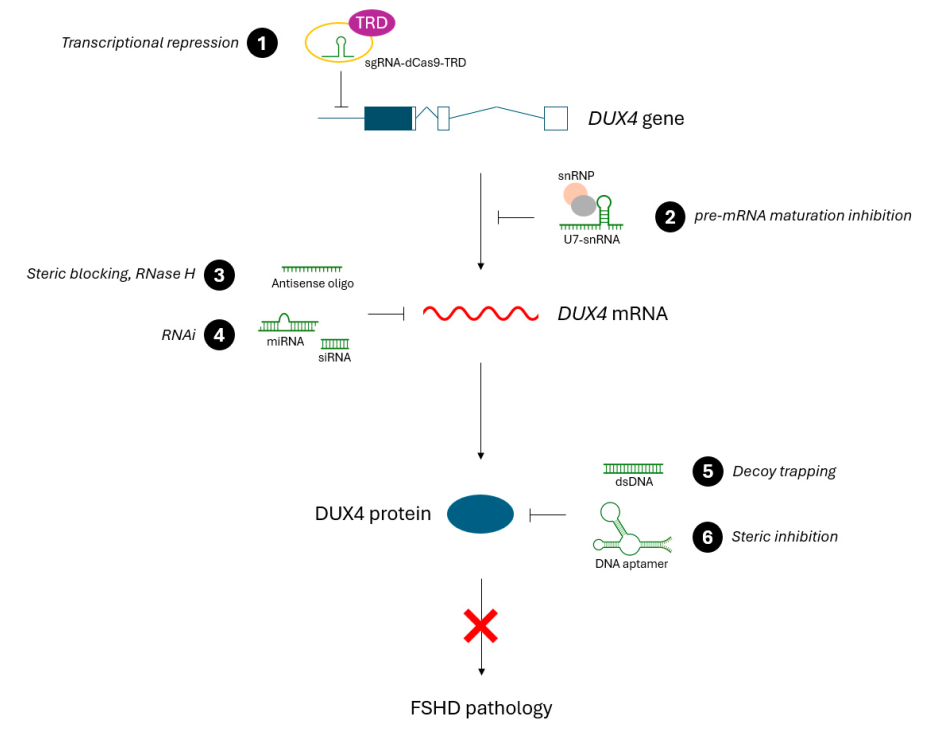
3. Oligonucleotide-Based Therapeutic Approaches
Since DUX4 is the primary driver of FSHD pathology, therapeutic strategies aim to inhibit its expression. Oligonucleotide-based approaches currently under investigation include:
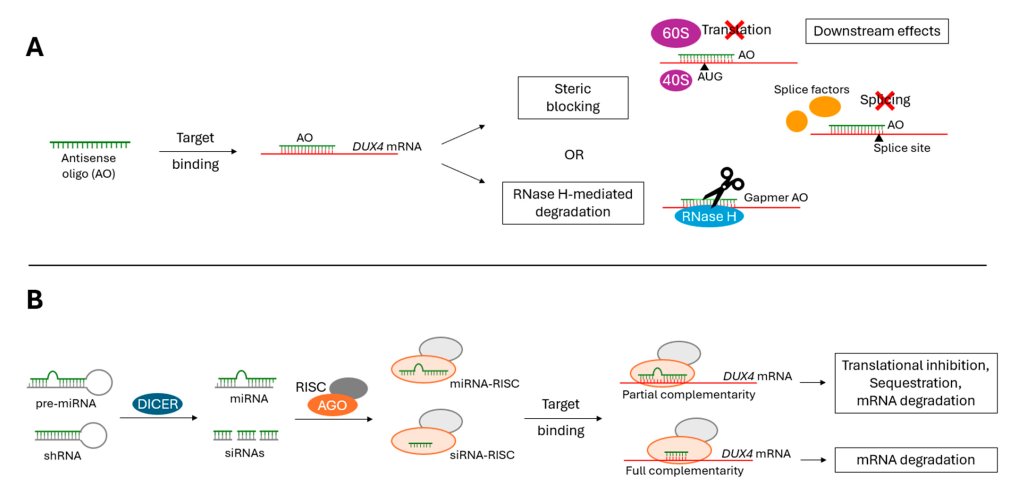
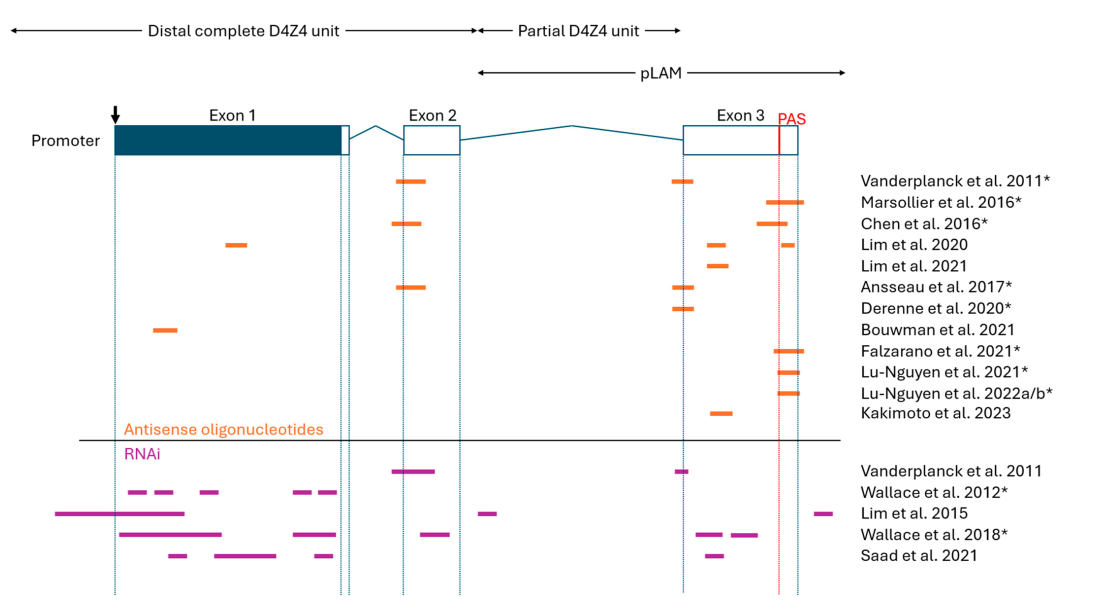
1. Antisense Oligonucleotides (ASOs)
- Designed to bind DUX4 mRNA and prevent translation.
- Can reduce DUX4 expression and alleviate muscle damage.
2. RNA Interference (RNAi)
- Uses siRNA molecules to degrade DUX4 mRNA.
- Has shown effectiveness in cell and animal models.
3. CRISPR/dCas9 Transcriptional Repression
- Uses a modified CRISPR system to silence the DUX4 gene.
- Represents a long-term therapeutic solution with high specificity.
4. Current Challenges and Future Prospects
While these therapies show promise, several challenges remain:
- Delivery barriers: Efficiently targeting skeletal muscles remains difficult.
- Long-term safety: The effects of chronic DUX4 suppression need further study.
- Immune response: Potential for off-target effects must be minimized.
5. Conclusion
Oligonucleotide-based therapies represent a groundbreaking approach to treating FSHD. Strategies such as ASOs, RNAi, and CRISPR/dCas9 have shown promise in preclinical studies. Further research is needed to refine these methods and bring them to clinical application.

| Published | 8/21/2024 |
| Address | https://doi.org/10.3390/ijms25169065 |
| Authors | Samuel L. Beck 1 and Toshifumi Yokota |














