1. Study Overview
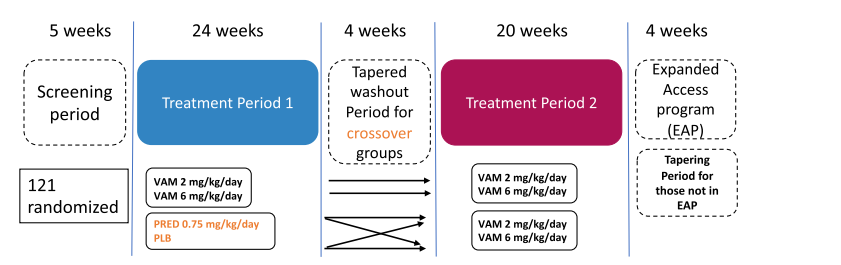
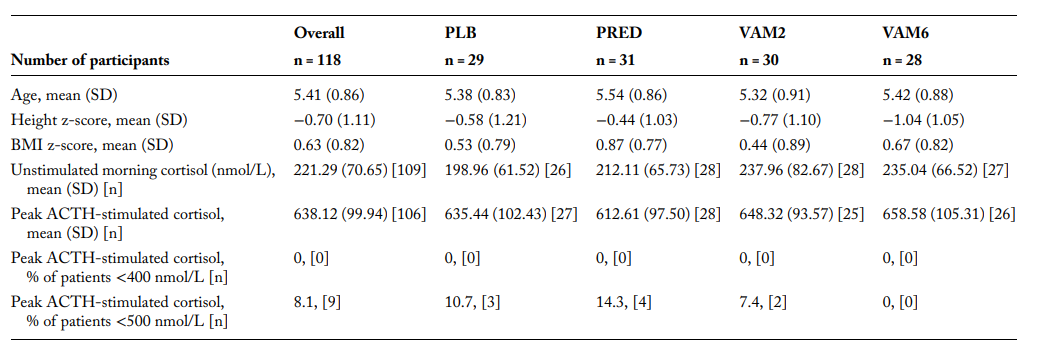
This Phase 2b clinical trial investigated the effects of vamorolone and prednisone on adrenal suppression (AS) in boys with Duchenne Muscular Dystrophy (DMD). The study included 121 steroid-naive boys aged 4 to under 7 years, all with genetically confirmed DMD.
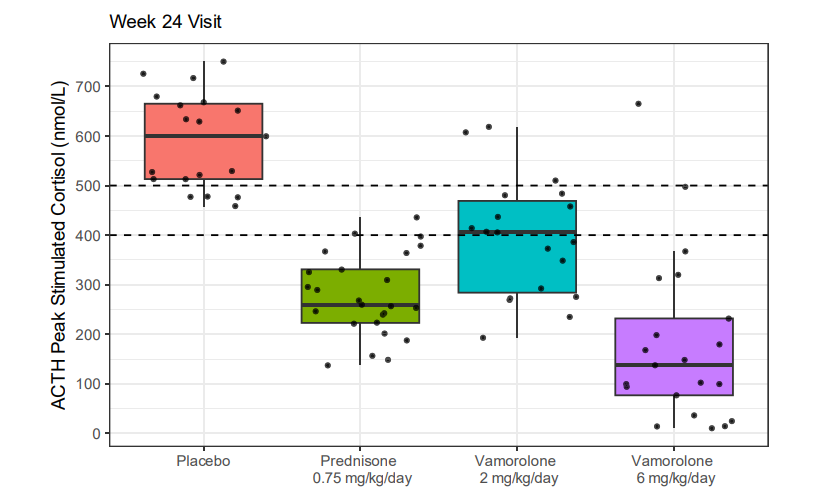
2. Methods and Adrenal Function Assessment
Adrenal function was assessed using:
- Morning cortisol levels
- ACTH-stimulated cortisol levels
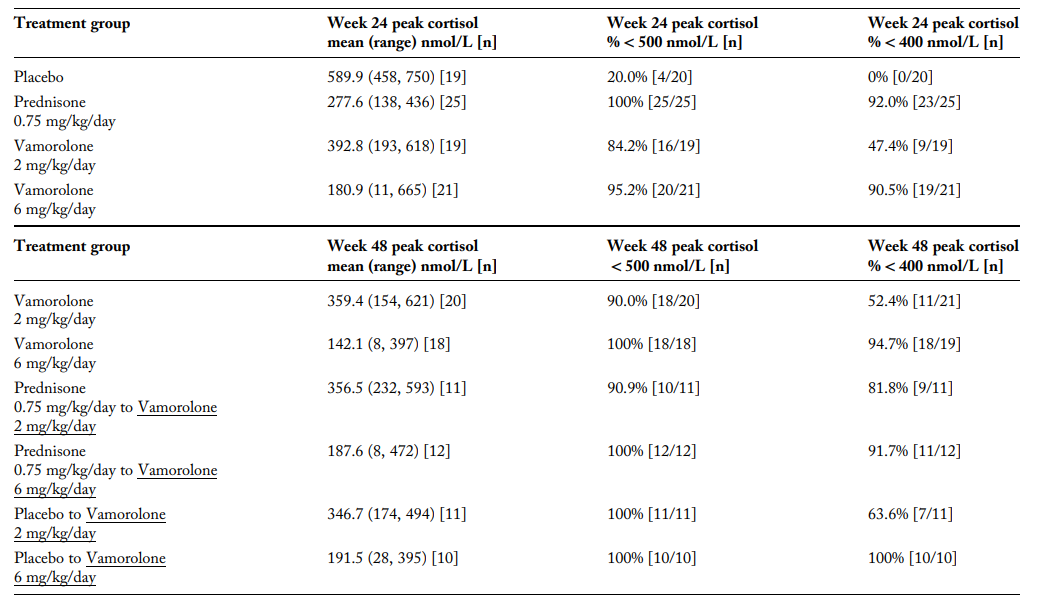
Adrenal suppression (AS) was defined using two different cortisol thresholds:
- Historical threshold: <500 nmol/L
- Revised threshold: <400 nmol/L (based on updated cortisol assays)
3. Key Findings
The study found that:
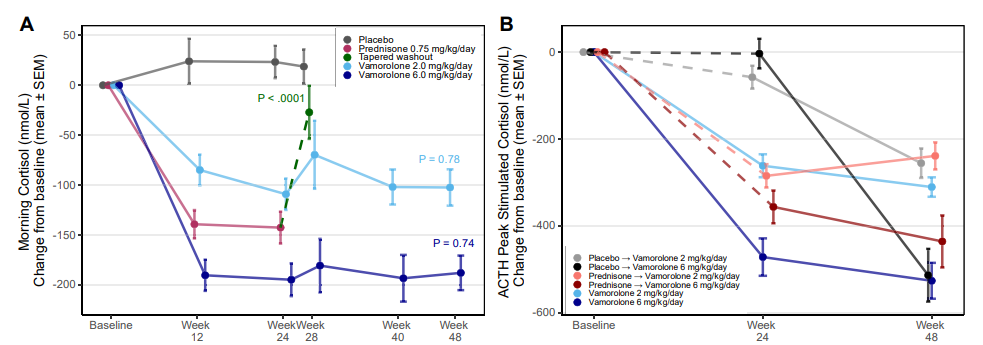
- Both vamorolone and prednisone caused significant adrenal suppression.
- The degree of suppression was dose-dependent for vamorolone.
- At 24 weeks:
- 95.2% of participants on vamorolone (6 mg/kg/day) showed AS.
- 100% of participants on prednisone showed AS.
- Some participants in the placebo group also showed AS, suggesting possible baseline adrenal dysfunction in DMD patients.
4. Clinical Implications
The results suggest that:
- Vamorolone may have fewer growth-related side effects compared to prednisone, but it still carries a significant risk of adrenal suppression.
- Monitoring adrenal function is essential for DMD patients receiving these treatments.
- A revised cortisol threshold (<400 nmol/L) may be more appropriate for assessing adrenal insufficiency.
5. Recommendations
Based on these findings, the study recommends:
- Using hydrocortisone for stress dosing in patients receiving vamorolone, especially during:
- Illness
- Surgery
- Other stress-related conditions
- Further research to refine cortisol thresholds for better management of adrenal health in pediatric patients.
6. Conclusion
This study underscores the importance of adrenal function monitoring in DMD patients receiving steroid treatments. While vamorolone may be a promising alternative to prednisone, it still carries risks of adrenal suppression. The findings highlight the need for further research to optimize treatment protocols and minimize adverse effects.








| Published | 8/4/2024 |
| Address | https://doi.org/10.1210/clinem/dgae521 |
| Authors | Alexandra Ahmet,1,2 Rebecca Tobin,3 Utkarsh J. Dang,3 Raoul Rooman,4 Michela Guglieri,5 Paula R. Clemens,6 Eric P. Hoffman,7 and Leanne M. Ward1,2; on behalf of the CINRG Vamorolone 004 Investigators |














