Overview
This paper explores the vital role of mitochondria in regulating muscle stem cells, particularly satellite cells (SCs), which are crucial for skeletal muscle regeneration. Mitochondria play a key role in energy production through oxidative phosphorylation (Oxphos), influencing SC fate, including their quiescence, activation, self-renewal, and differentiation.
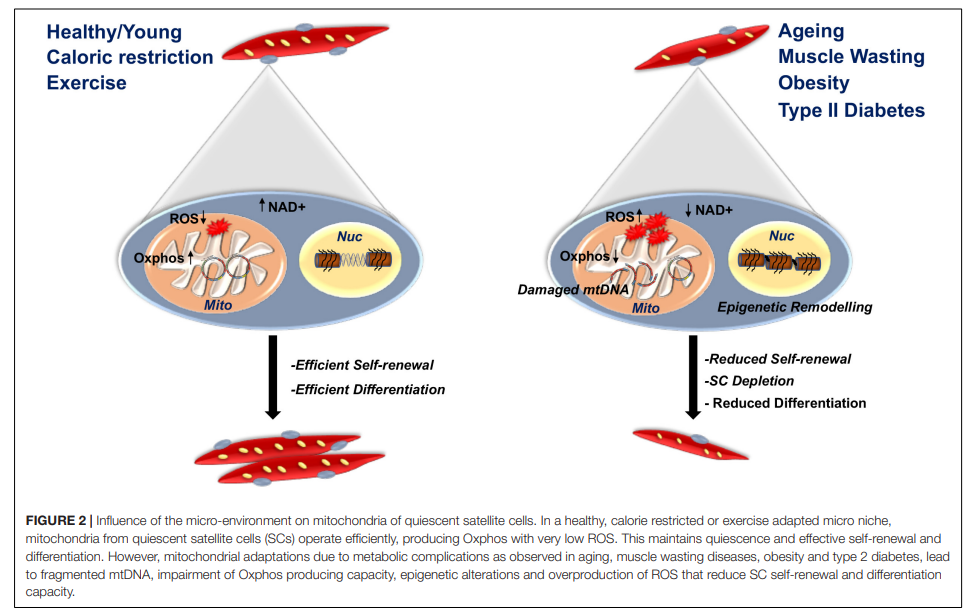
Mitochondrial Adaptation and Satellite Cell Function
- Quiescent SCs: Rely on fatty acid oxidation for energy maintenance.
- Activated SCs: Shift to glycolysis to meet the high energy demands of proliferation.
- Differentiating SCs: Increase Oxphos capacity to support muscle repair.
- Mitochondrial Reactive Oxygen Species (ROS): Moderate levels influence SC fate, but excessive ROS can be detrimental to cell function.
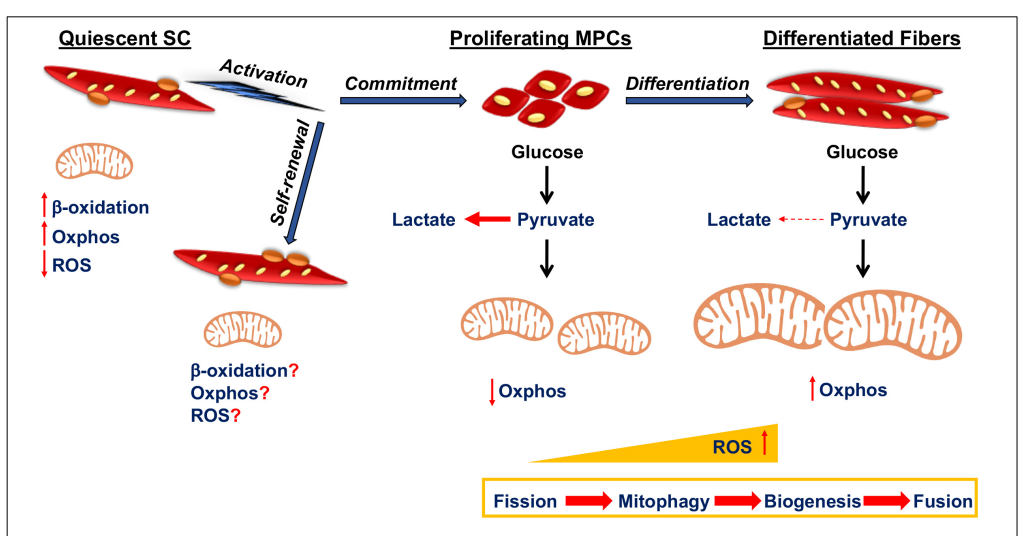
Mitochondrial Dynamics and Muscle Stem Cell Function
- Mitochondrial Biogenesis: Essential for SC differentiation.
- Fission and Fusion: Regulates SC energy balance and regeneration capacity.
- Mitophagy: Removes damaged mitochondria to ensure efficient differentiation.
- Aging and Disease Impact: Mitochondrial dysfunction in muscle-wasting diseases leads to poor regeneration.
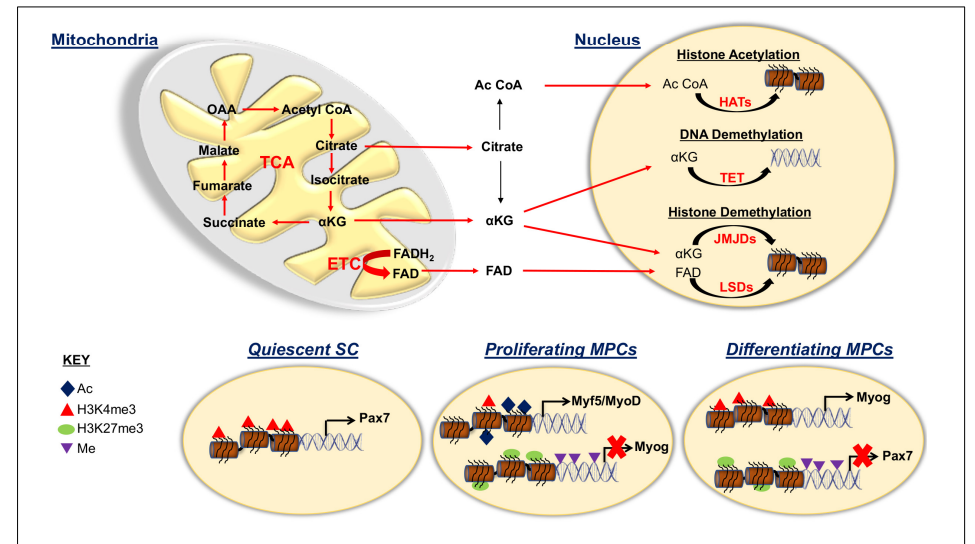
Mitochondria, Epigenetics, and Muscle Repair
Understanding how mitochondria interact with epigenetic factors is crucial for regulating SC fate. TCA cycle intermediates influence epigenetic modifications, which play a key role in muscle repair.
Potential Interventions for Muscle Regeneration
- Exercise: Enhances mitochondrial function and improves SC efficacy.
- Caloric Restriction: Supports mitochondrial health and delays aging effects.
- Pharmacological Approaches: Developing mitochondria-targeted therapies for improving muscle regeneration.
Conclusion and Future Directions
This study highlights the crucial role of mitochondrial metabolism in regulating muscle stem cells and its implications for skeletal muscle regeneration. Understanding how environmental factors affect SC mitochondrial function could lead to novel therapeutic strategies. Further research is needed to uncover how mitochondrial signals can be harnessed to enhance muscle repair.


| Published | 6/16/2020 |
| Address | doi: 10.3389/fcell.2020.00480 |
| Authors | Debasmita Bhattacharya, Anthony Scimè |














