1. Introduction: Eteplirsen and Exon 51 Skipping
Eteplirsen is a phosphorodiamidate morpholino oligomer (PMO) designed to restore **dystrophin production** in patients with **Duchenne muscular dystrophy (DMD)** amenable to **exon 51 skipping**. This study evaluates the **safety, tolerability, and pharmacokinetics** of eteplirsen in young boys aged **6 to 48 months**.
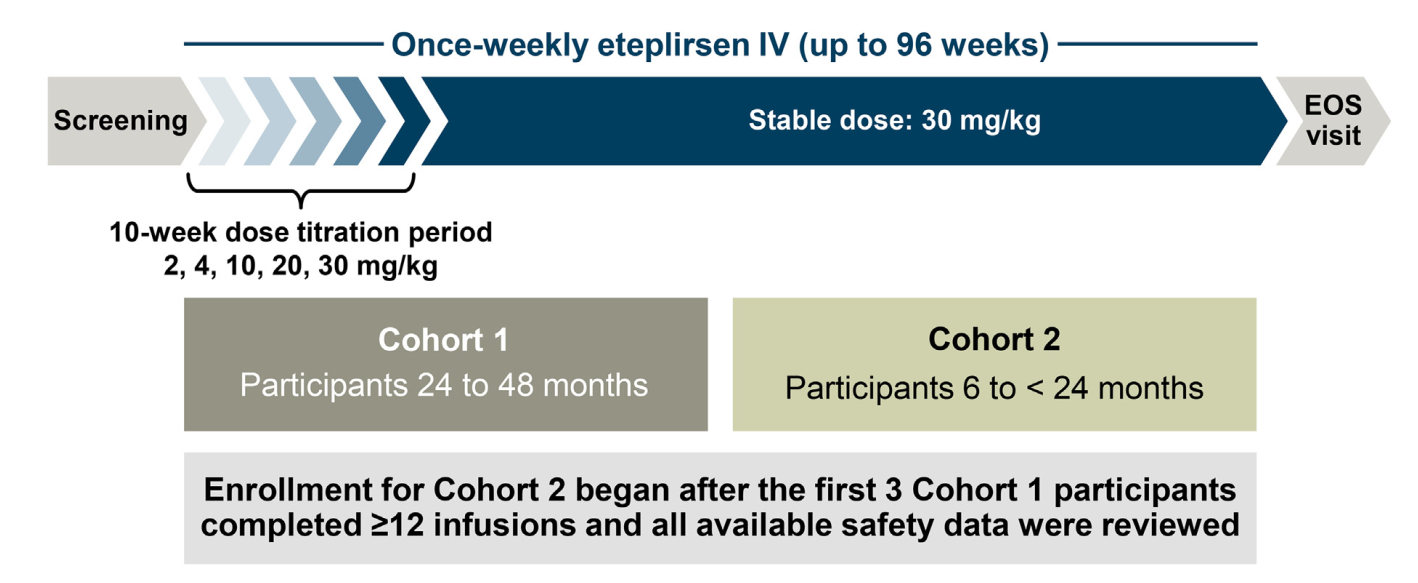
2. Study Design: Open-Label, Dose-Escalation Trial
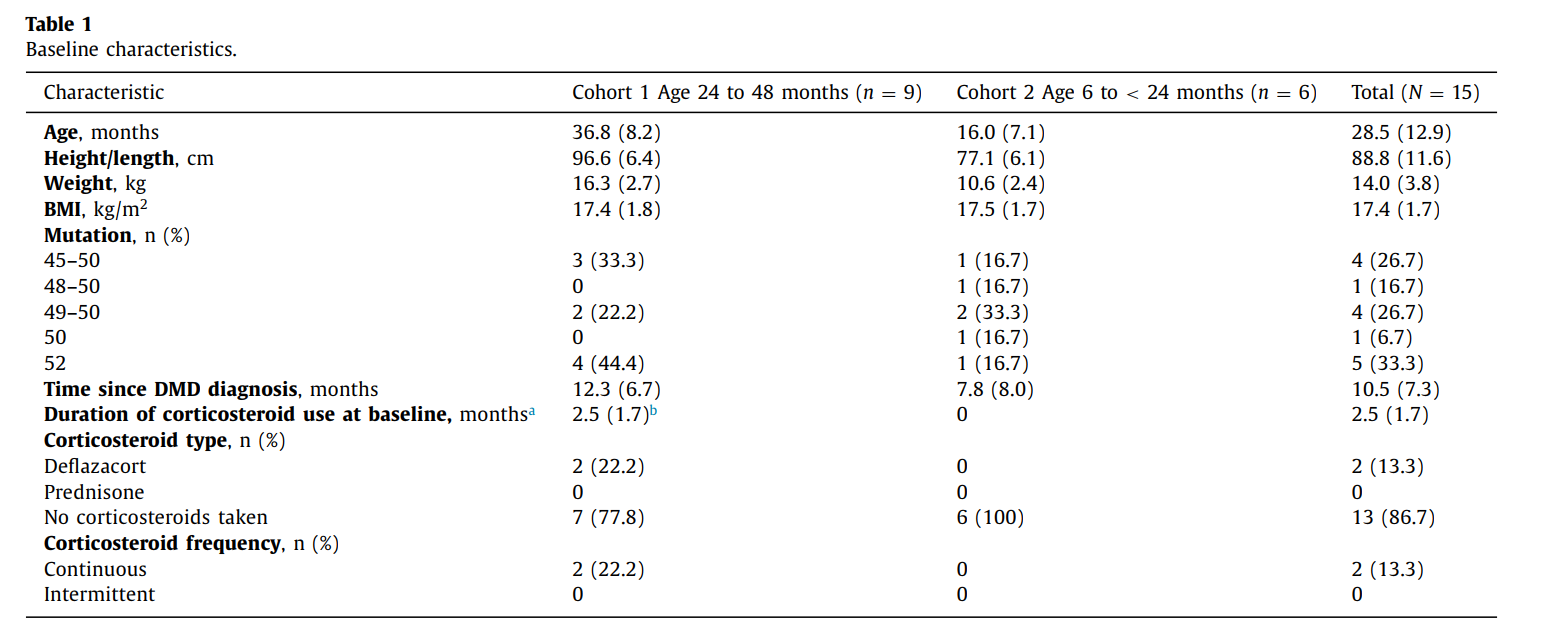
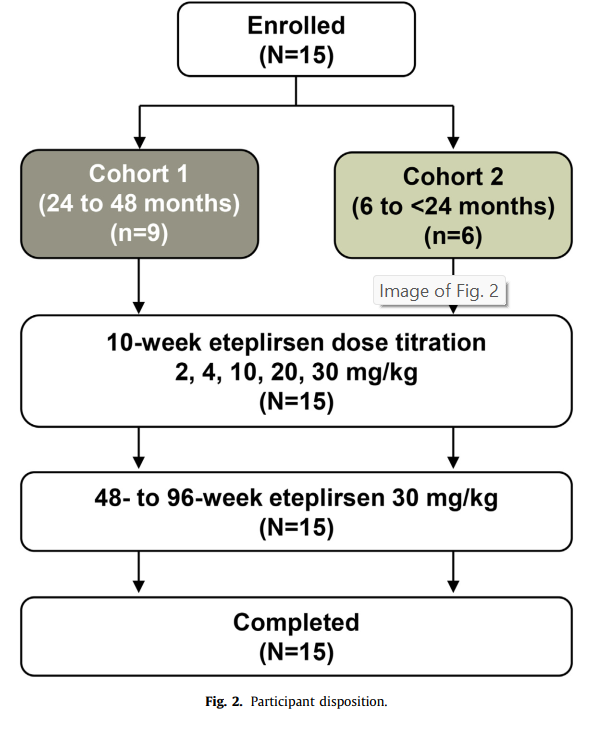
- Participants: 15 boys divided into two age cohorts:
- 24 to 48 months group.
- 6 to 24 months group.
- Treatment: Weekly intravenous **eteplirsen infusions** with doses increasing from **2 mg/kg to 30 mg/kg** over **96 weeks**.
3. Primary Outcome: Safety and Tolerability
**Most treatment-emergent adverse events (TEAEs) were mild**, with the most common including:
- Pyrexia (**fever**).
- Cough and nasopharyngitis (**common cold symptoms**).
- Gastrointestinal symptoms (**vomiting, diarrhea**).
Importantly, there were **no treatment-related discontinuations** and **no evidence of kidney toxicity**.
4. Pharmacokinetics: Consistency with Older Patients
- Eteplirsen was **well-absorbed**, with predictable distribution.
- Findings were **consistent with previous studies** in older children.
- Supportive evidence for **safe use in younger DMD patients**.
5. Conclusion: Expanding the Treatment Age Range
This study confirms that **eteplirsen is safe and well-tolerated** even in **very young boys with DMD**. However, as this trial was not designed to measure **efficacy**, **ongoing studies** are needed to evaluate **long-term benefits** and further refine **its safety profile**.








| Published | 22 March 2023 |
| Address | https://doi.org/10.1016/j.nmd.2023.03.008 |
| Authors | E. Mercuri a,b, A.M. Seferian c, L. Servais c,d,e, N. Deconinck f,g, H. Stevenson h, X. Ni h,W. Zhang h, L. East h, S. Yonren h, F. Muntoni i,j,∗, 4658-102 Study Group |














