Introduction
Nusinersen is an FDA-approved drug for treating Spinal Muscular Atrophy (SMA). While its benefits in infants and young children have been well-documented, its effectiveness in adolescents and adults remains an area of active research. This study systematically evaluates its impact on motor function in patients aged 13 to 72 years.
Study Design and Patient Population
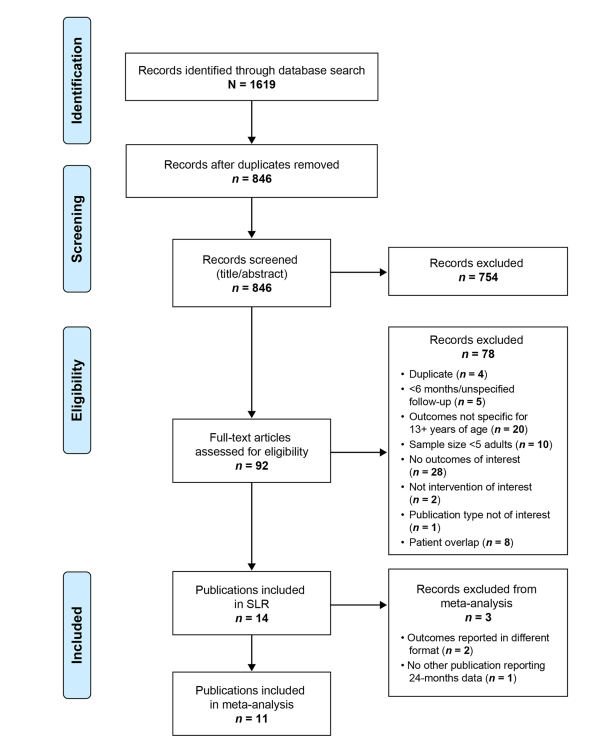
- Systematic review and meta-analysis of 14 studies.
- Total of 539 patients with SMA Type II or III.
- Age range: 13–72 years.
- Follow-up period: Up to 24 months.
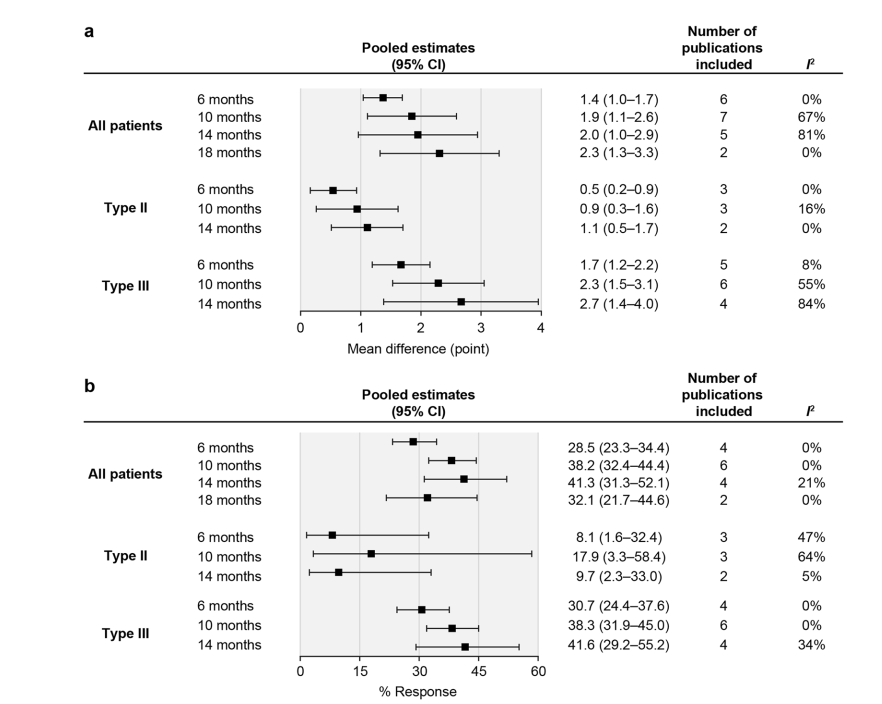
Key Findings: Motor Function Improvements
1. Hammersmith Functional Motor Scale–Expanded (HFMSE)
- Average increase: 2.3 points.
- Clinically meaningful improvement: 32.1% of patients at 18 months.
2. Revised Upper Limb Module (RULM)
- Average increase: 1.1 points.
- Clinically meaningful improvement: 38.3% of patients at 14 months.
3. Six-Minute Walk Test (6MWT) in Ambulatory Patients
- Average increase: 25.0 meters.
- Improvement observed at 14 months.
Implications for SMA Management
These findings support the use of Nusinersen in adolescents and adults with SMA. The study emphasizes that:
- Younger patients tend to show greater motor improvements.
- Disease severity influences the extent of response to treatment.
- Longer follow-up is needed to assess long-term benefits.
Conclusion
This systematic review and meta-analysis confirm that Nusinersen is effective in stabilizing or improving motor function in adolescents and adults with SMA. The findings highlight the need for personalized treatment approaches based on disease severity and functional capacity.



| Published | 7/29/2024 |
| Address | https://doi.org/10.1007/s40120-024-00653-2 |
| Authors | Tim Hagenacker · Lorenzo Maggi · Giorgia Coratti · Bora Youn · Stephanie Raynaud · Angela D. Paradis · Eugenio Mercuri |














