The paper presents findings from a Phase 3 clinical trial (EMBARK) evaluating the efficacy and safety of a gene therapy, delandistrogene moxeparvovec, in patients with Duchenne muscular dystrophy (DMD). DMD is a severe, inherited condition that progressively weakens muscles due to the absence of dystrophin. The therapy uses an AAV vector to deliver a functional dystrophin gene into muscle cells. The trial involved boys aged 4 to 8, who were randomized to receive either the therapy or a placebo.
Primary Study Objective
The primary objective was to assess improvements in motor function using the North Star Ambulatory Assessment (NSAA) after 52 weeks. Although the therapy group showed slight improvement compared to the placebo group, the difference was not statistically significant.
Secondary Outcomes
Secondary measures included micro-dystrophin expression and timed motor tests such as Time to Rise and 10-meter Walk/Run. These tests showed some favorable results for the treatment group.
Safety Data
Safety data indicated that most side effects were mild to moderate, with no deaths or life-threatening events. However, seven patients experienced serious adverse events related to the treatment, but these were resolved.
Conclusion
Overall, the therapy was well tolerated, but its efficacy remains uncertain at this stage of the trial. Further long-term studies are needed to confirm its potential benefits for DMD patients.
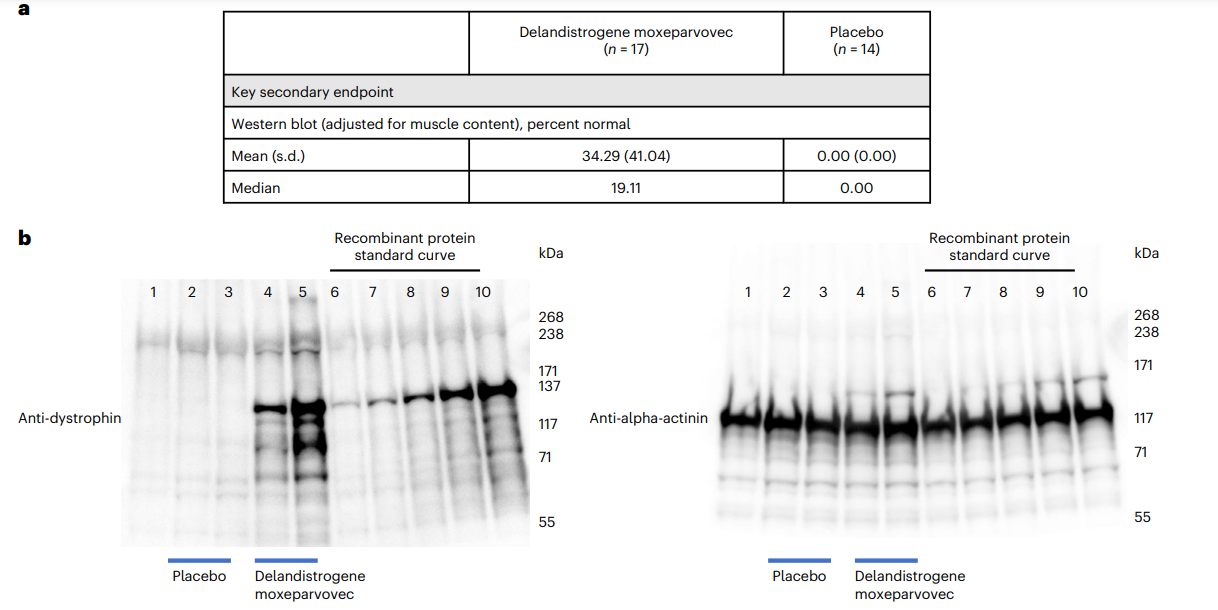
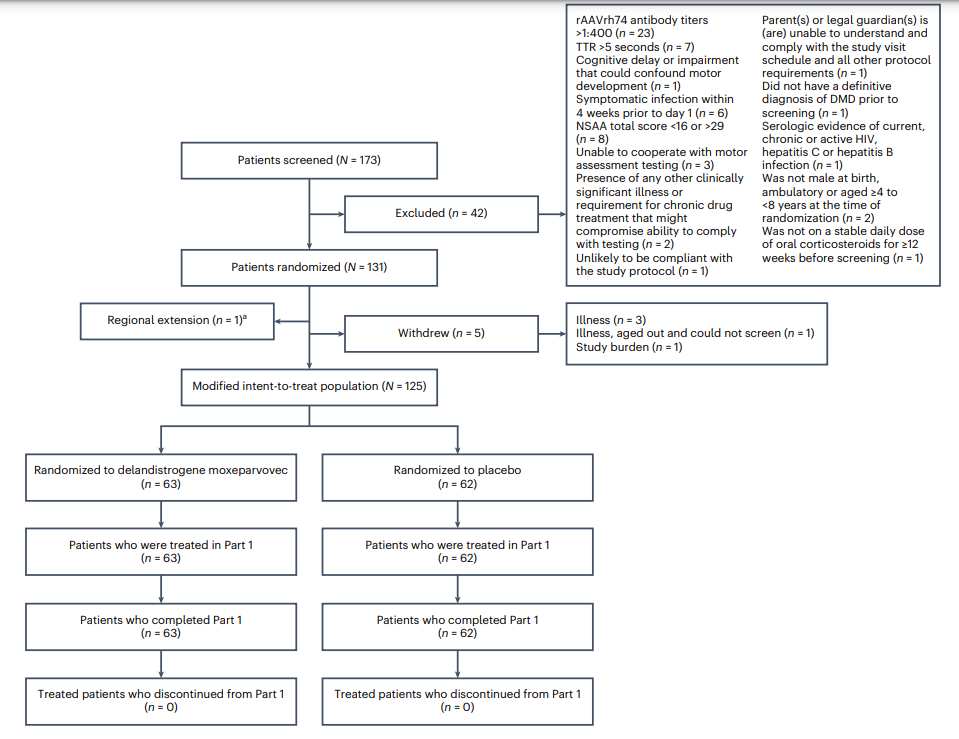







| Authors | Jerry R. Mendell, Francesco Muntoni, Craig M. McDonald, Eugenio M. Mercuri, Emma Ciafaloni, Hirofumi Komaki, Carmen Leon-Astudillo, Andrés Nascimento, Crystal Proud, Ulrike Schara-Schmidt, Aravindhan Veerapandiyan, Craig M. Zaidman, Maitea Guridi, Alexander P. Murphy, Carol Reid, Christoph Wandel, Damon R. Asher, Eddie Darton, Stefanie Mason, Rachael A. Potter, Teji Singh, Wenfei Zhang, Paulo Fontoura, Jacob S. Elkins, Louise R. Rodino-Klapac |
| Corresponding Author | Jerry R. Mendell (JMendell@Sarepta.com) |
| Article Title | AAV Gene Therapy for Duchenne Muscular Dystrophy: The EMBARK Phase 3 Randomized Trial |
| Publication Date | Accepted: 17 September 2024 |
| Journal Name | Nature Medicine |
| Journal Ranking | Q1 (Quartile 1) |
| Keywords | Duchenne muscular dystrophy, gene therapy, micro-dystrophin, clinical trial, delandistrogene moxeparvovec |
| Methods Used | Randomized, double-blind, placebo-controlled trial; North Star Ambulatory Assessment, Western blot for micro-dystrophin |
| DOI | https://doi.org/10.1038/s41591-024-03304-z |














