What is Casimersen?
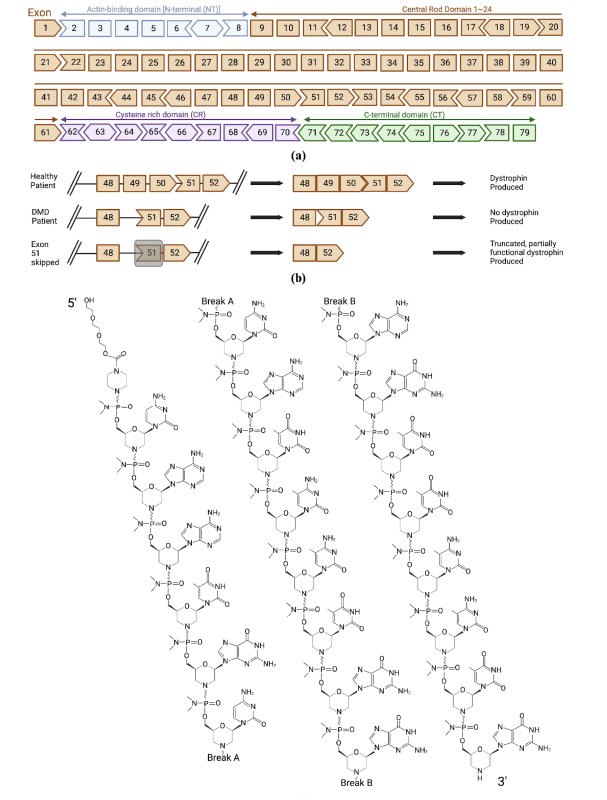
Casimersen (AMONDYS 45™) is an antisense oligonucleotide (ASO) designed to treat Duchenne Muscular Dystrophy (DMD) patients with a genetic mutation amenable to exon 45 skipping. DMD is a severe neuromuscular disorder caused by mutations in the dystrophin gene, leading to progressive muscle degeneration. Casimersen helps restore dystrophin production by altering the pre-mRNA splicing process, resulting in a shorter yet functional dystrophin protein.
Mechanism of Action
Casimersen works by binding to exon 45 of the dystrophin pre-mRNA, allowing the splicing machinery to skip this exon. This modification restores the reading frame, leading to the production of a shorter but partially functional dystrophin protein, which stabilizes muscle fibers and slows disease progression.
FDA Approval and Clinical Trials
The U.S. Food and Drug Administration (FDA) granted accelerated approval for Casimersen in February 2021 based on its ability to increase dystrophin levels in patients. However, full approval depends on ongoing Phase III clinical trials to confirm its long-term clinical benefits.
Key Findings from the ESSENCE Trial
The ESSENCE trial evaluated Casimersen's efficacy in increasing dystrophin expression. Results showed a statistically significant increase in dystrophin levels compared to placebo, reinforcing its potential as an effective therapy for exon 45 mutations in DMD patients.
Side Effects and Safety Considerations
Casimersen is generally well tolerated, but common side effects include:
- Upper respiratory tract infections
- Headaches
- Injection site reactions
Monitoring for potential long-term kidney effects is ongoing to ensure the drug's safety profile.
Conclusion and Future Outlook
While Casimersen is not a cure, it represents a crucial step in treating DMD, particularly for the ~8% of patients with exon 45 mutations. Ongoing clinical trials aim to validate its long-term impact, bringing hope to the DMD community.


| Information | Details |
| Authors | Milyard Assefa, Addison Gepfert, Meesam Zaheer, Julia M. Hum, Brian W. Skinner |
| Corresponding Author | Julia M. Hum |
| Article Title | Casimersen (AMONDYS 45™): An Antisense Oligonucleotide for Duchenne Muscular Dystrophy |
| Publication Date | 20-Apr-24 |
| Journal Name | Biomedicines |
| Keywords | AMONDYS 45, antisense oligonucleotide, casimersen, Duchenne muscular dystrophy, dystrophin |
| Methods Used | Exon skipping, clinical trials (Phase III), dystrophin quantification, immunofluorescence |
| DOI | 10.3390/biomedicines12040912 |














