Duchenne muscular dystrophy (DMD) is a fatal neuromuscular disease caused by mutations in the dystrophin gene. Approximately 10-15% of cases result from nonsense mutations (nmDMD), which ataluren targets to restore functional dystrophin. This review provides clinical guidelines for ataluren use and proposes an implementation model in Sweden.
Methodology
The study conducted a targeted review of literature (1995-2018), including clinical trials, guidelines, and expert commentaries on nmDMD and ataluren. The focus was on treatment initiation, monitoring, and discontinuation criteria.
Clinical Outcomes
Ataluren has shown efficacy in delaying muscle degeneration, preserving ambulation, and improving pulmonary function in ambulatory nmDMD patients. Treatment is recommended from early diagnosis (ages 3-5) and continued until key milestones, such as reduced pulmonary capacity or significant loss of upper limb function.
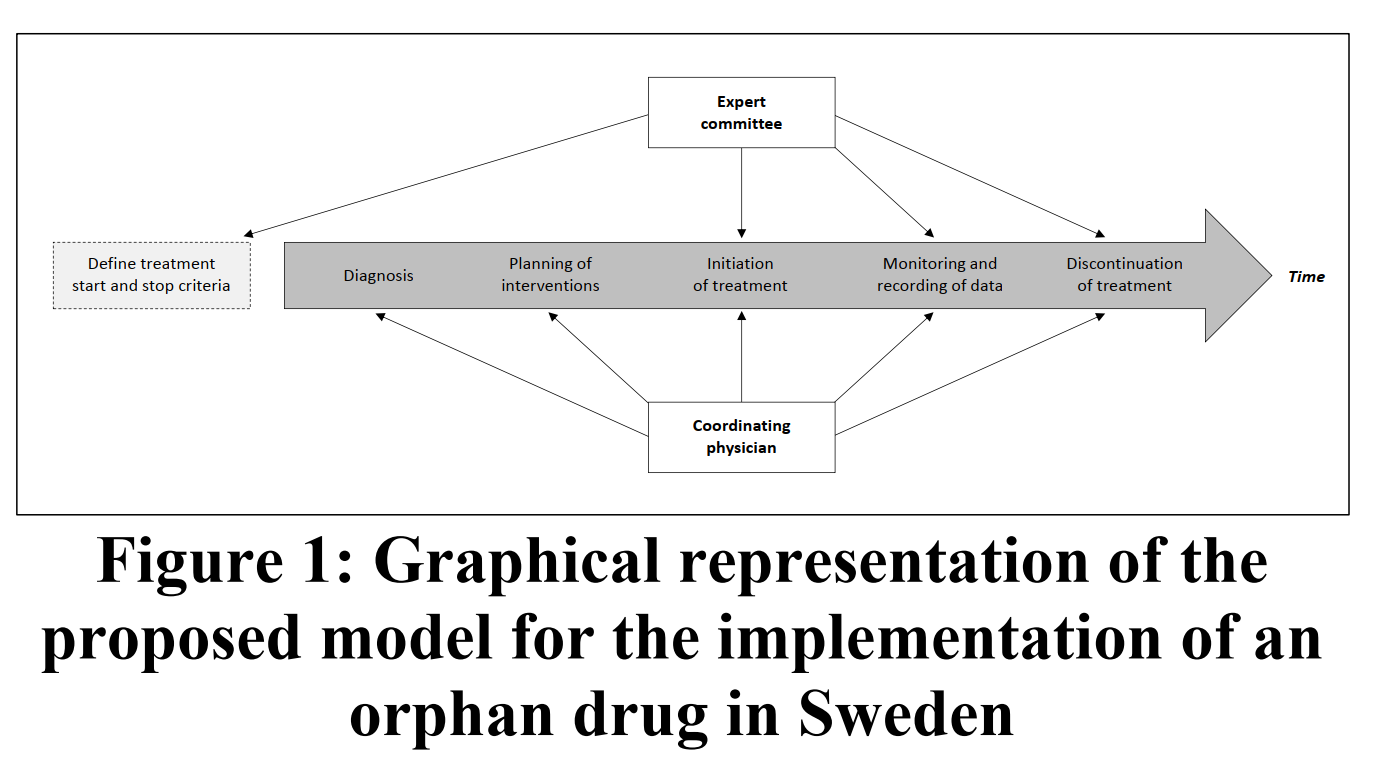
Proposed Implementation in Sweden
The study suggests a centralized expert committee to ensure equitable access, proper monitoring, and optimal use of ataluren in Sweden. Multidisciplinary teams would oversee patient care and provide additional clinical evaluations.
Economic and Policy Challenges
High costs and variations in reimbursement policies across countries complicate ataluren's adoption. Comprehensive economic evaluations must consider caregiver burdens and societal impacts.
Conclusion
These recommendations aim to enhance access to ataluren and similar treatments in Sweden, ensuring better outcomes for patients with rare neuromuscular diseases.



| Field | Details |
| Authors | Erik Landfeldt, Thomas Sejersen, Már Tulinius |
| Corresponding Author | Erik Landfeldt |
| Article Title | A Mini Review and Implementation Model for Using Ataluren to Treat Nonsense Mutation Duchenne Muscular Dystrophy |
| Publication Date | Accepted, exact publication date unavailable |
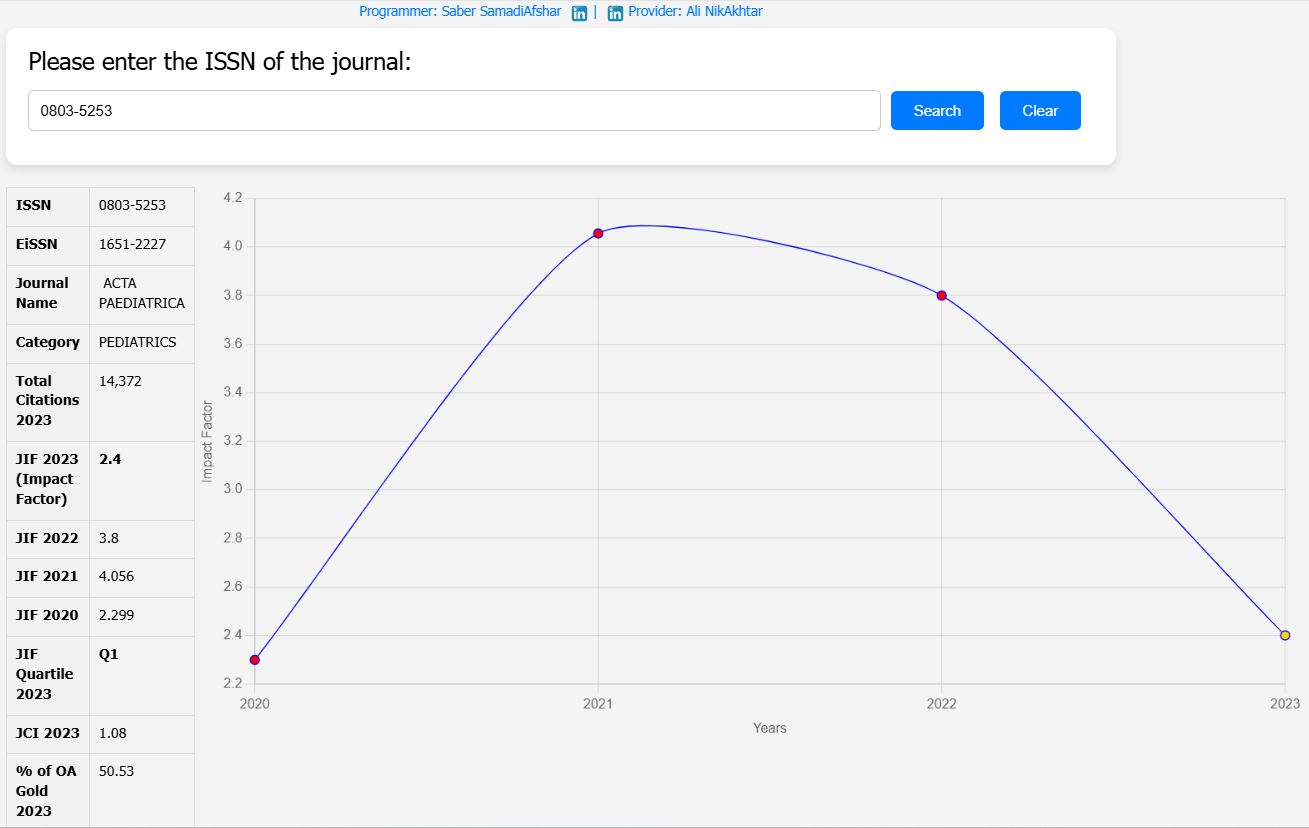
| Journal Name | Acta Paediatrica |
| Keywords | Ataluren, Duchenne muscular dystrophy, implementation model, treatment guidelines, Sweden |
| Methods Used | Literature review (1995-2018), including randomized trials, guidelines, and clinical commentaries |
| DOI | 10.1111/apa.14568 |