1. Introduction
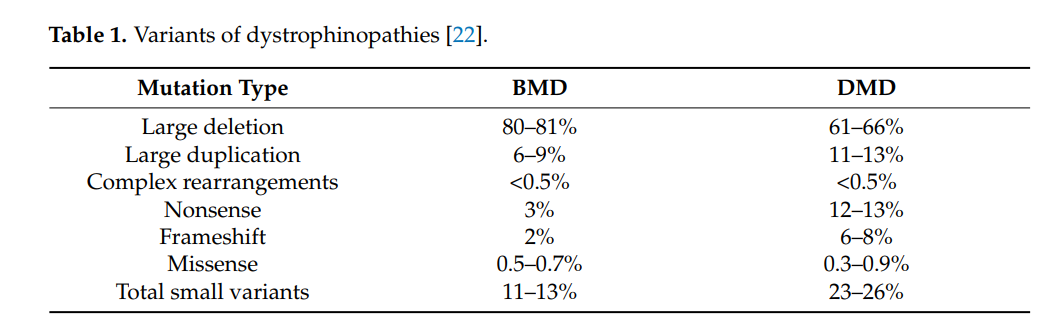
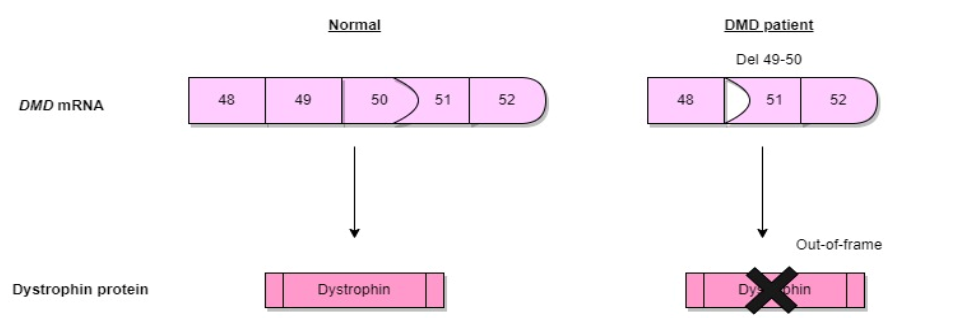
Duchenne Muscular Dystrophy (**DMD**) is a severe **genetic disorder** caused by **mutations in the dystrophin gene**, leading to **muscle degeneration** and **progressive weakness**. In recent years, **exon skipping therapy** has emerged as a promising **molecular treatment**, utilizing **antisense oligonucleotides (AONs)** to **restore the reading frame** of the dystrophin gene. This therapy aims to **convert the severe DMD phenotype** into a **milder Becker muscular dystrophy (BMD) form**.
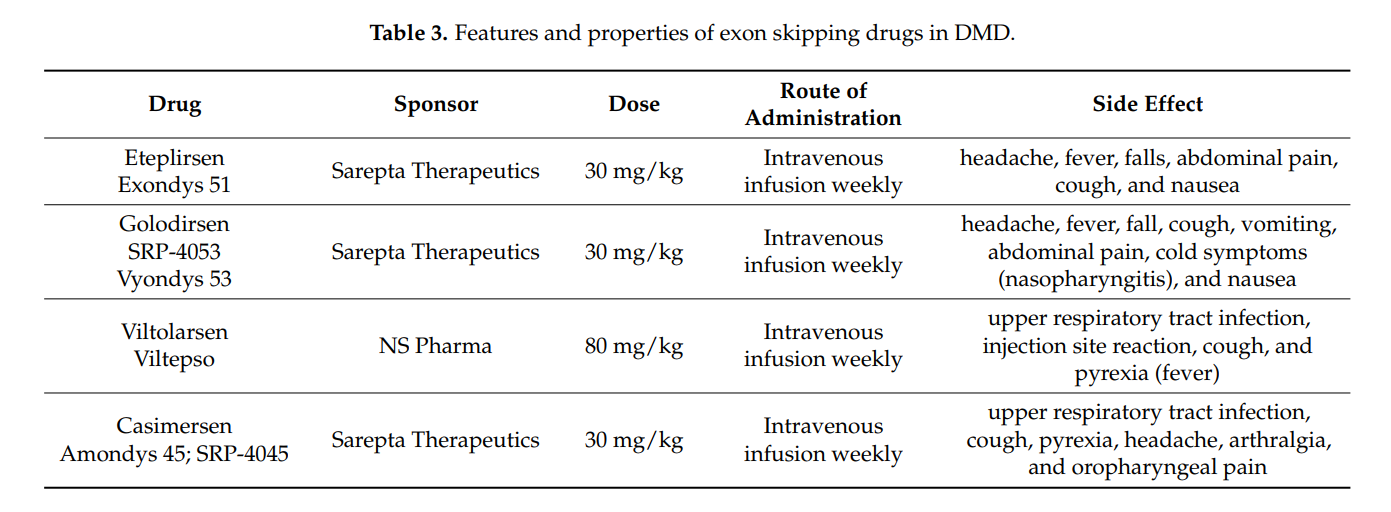
2. FDA-Approved Exon Skipping Therapies
- Eteplirsen (Exon 51) – The first exon-skipping drug approved by the FDA.
- Golodirsen (Exon 53) – Another exon-skipping drug approved for specific DMD mutations.
- Viltolarsen (Exon 53) – Approved alongside Golodirsen for exon 53 skipping.
- Casimersen (Exon 45) – Recently approved for patients with exon 45 mutations.
3. Mechanism of Action
Exon skipping works by **using AONs to bind specific mRNA regions**, hiding the targeted exon from the **splicing machinery**, allowing the production of a **functional but shorter dystrophin protein**. Advances in **AON delivery** and **stability enhancement** have led to chemical modifications such as:
- 2′-O-methyl phosphorothioate (2OMePS) – Enhances AON stability and efficiency.
- Phosphorodiamidate morpholino oligomers (PMOs) – Improves drug delivery to muscle cells.
4. Challenges and Limitations
- **Low dystrophin restoration levels** – Current therapies only produce small amounts of functional dystrophin.
- **Frequent intravenous infusions** – Most treatments require **weekly administration**, which is burdensome.
- **Limited effect on heart function** – The impact of exon skipping on **cardiac muscle** remains unclear.
- **High cost and accessibility issues** – These therapies remain expensive and are not widely available.
5. Future Directions
Despite these challenges, **ongoing research** is exploring **next-generation exon skipping therapies**, improving **AON efficiency**, and **developing multi-exon skipping approaches**. These advancements could enhance **treatment outcomes** and provide **better therapeutic options for DMD patients**.

| Published | 7/14/2022 |
| Address | https://doi.org/10.3390/genes13071241 |
| Authors | Gökçe Eser and Haluk Topalo˘glu |














