Myotonic Dystrophy (DM): Causes, Symptoms, and Research Advances
What is Myotonic Dystrophy?
Myotonic Dystrophy (DM) is a genetic disorder that affects muscle function and multiple body systems. It is inherited in an autosomal dominant pattern, meaning a person only needs to inherit one copy of the altered gene to develop the condition.
Types of Myotonic Dystrophy
1. Myotonic Dystrophy Type 1 (DM1) - Steinert's Disease
DM1 is caused by a mutation in the DMPK gene (Dystrophia Myotonica Protein Kinase). This mutation involves an expansion of CTG trinucleotide repeats, leading to symptoms such as:
- Myotonia: Prolonged muscle contractions.
- Muscle Weakness and Wasting: Progressive loss of muscle strength.
- Systemic Complications: Including heart arrhythmias, cataracts, and endocrine disorders.
- Congenital DM1: A severe form affecting infants, causing breathing difficulties and delayed development.
2. Myotonic Dystrophy Type 2 (DM2)
DM2 is caused by a mutation in the CNBP gene (CCHC-Type Zinc Finger Nucleic Acid Binding Protein). The mutation consists of an expansion of CCTG repeats. Symptoms are generally milder than in DM1 but include:
- Muscle Weakness: Primarily affecting the legs and hips.
- Myotonia: Delayed muscle relaxation.
- Fatigue and Pain: Common in affected individuals.
- Heart and Endocrine Issues: Similar to DM1 but less severe.
Current Treatments and Supportive Care
While there is no cure for myotonic dystrophy, supportive treatments help manage symptoms:
- Cardiac Monitoring: Pacemakers or defibrillators for arrhythmias.
- Physical Therapy: Maintains mobility and reduces stiffness.
- Medications: Sodium channel blockers like mexiletine to manage myotonia.
- Respiratory Support: Non-invasive ventilation for breathing difficulties.
Future Research and Emerging Therapies
Scientists are actively researching gene therapy and molecular treatments to address the root causes of DM. Some promising approaches include:
- Antisense Oligonucleotides (ASO): Targeting toxic RNA to restore normal gene function.
- CRISPR Gene Editing: Investigated for correcting repeat expansions.
- Small Molecule Drugs: Aiming to modulate RNA splicing defects.
Conclusion
While no cure currently exists, advancements in gene therapy and targeted treatments offer hope for improving quality of life and potentially treating Myotonic Dystrophy in the future.
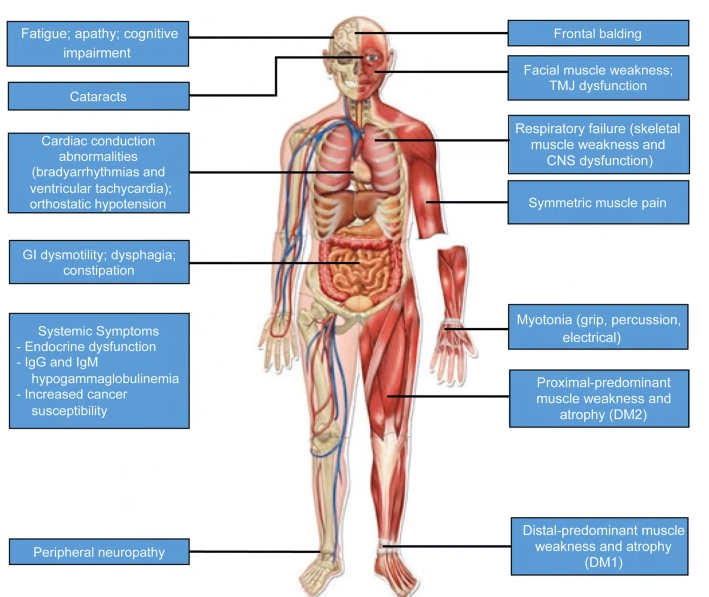
Figure: https://doi.org/10.1007/s13311-018-00679-z














