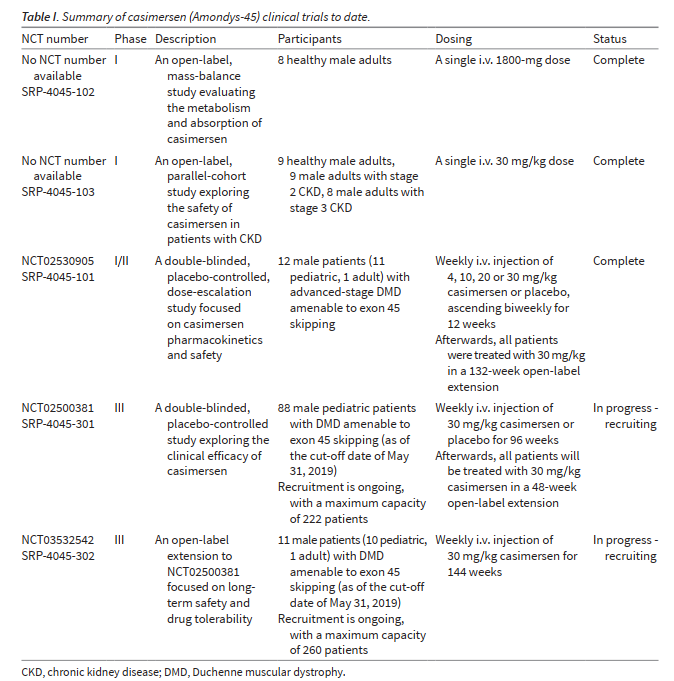
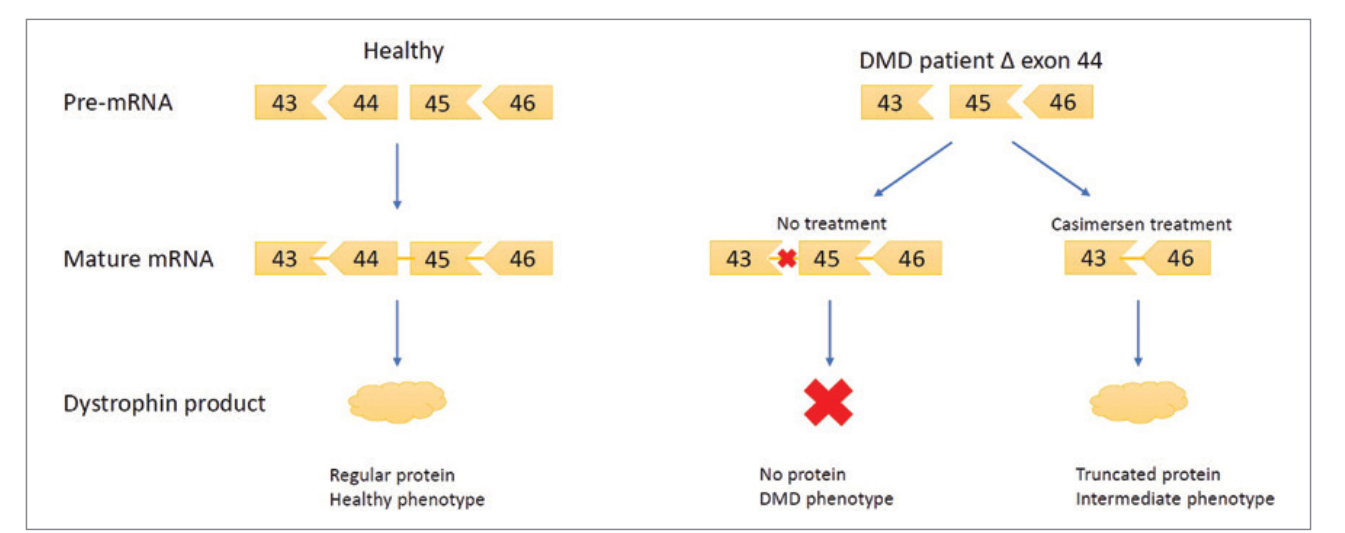
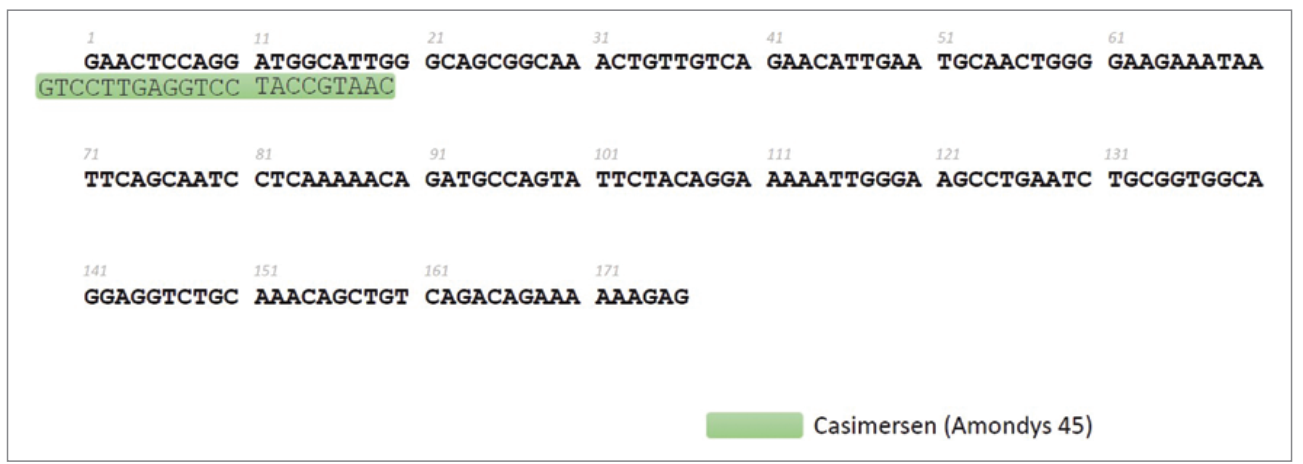
Duchenne Muscular Dystrophy (DMD) is a severe genetic disorder affecting males, characterized by progressive muscle degeneration and early death. Antisense-mediated exon skipping therapy offers new hope by producing a truncated but functional dystrophin protein. Casimersen (Amondys 45) specifically targets exon 45 of the dystrophin gene, which applies to about 8% of DMD patients. The drug was approved by the FDA in 2021, based on promising preclinical and phase I/II clinical trial results.
Phase III Clinical Trial (ESSENCE)
The ESSENCE trial is ongoing to further evaluate casimersen's effectiveness and safety. In preclinical trials, the drug showed stable pharmacokinetics, with low risk of drug-drug interactions. However, it raised concerns about kidney toxicity in animal models, which are being closely monitored in human trials.
Clinical Trial Results
In clinical trials, casimersen significantly improved dystrophin production compared to the placebo. No severe adverse effects were directly associated with the drug. Common side effects include mild respiratory infections, headaches, and joint pain.
Administration and Future Considerations
Casimersen is administered via weekly intravenous injections. Its continued approval depends on the ongoing phase III trial outcomes, which will determine its long-term efficacy and safety profile.


| Authors | H. Wilton-Clark, Toshifumi Yokota |
| Corresponding Author | Toshifumi Yokota |
| Article Title | Casimersen for Duchenne muscular dystrophy |
| Publication Date | 1-Dec-21 |
| Journal Name | Drugs of Today (Barcelona, Spain: 1998) |
| Journal Ranking | Not mentioned |
| Keywords | Casimersen, Amondys-45, Exon skipping therapy, Duchenne muscular dystrophy, Antisense therapy, Gene therapy |
| Methods Used | Preclinical trials, phase I/II clinical trials, phase III clinical trial (ongoing), RT-PCR, Western blot |
| DOI | 10.1358/dot.2021.57.12.3352740 |














