more
Impaired Glucose Tolerance in Adults with Duchenne and Becker Muscular Dystrophy

Abstract This study aimed to evaluate glucose tolerance in adult males with Becker muscular dystrophy (BMD) and Duchenne muscular dystrophy (DMD) using an oral glucose tolerance test (OGTT). The study investigated whether body composition influences glucose response. Participants: 28 adults with dystrophinopathy (13 BMD, 15 DMD) and 12 non-dystrophic controls. Method: 75g glucose ingestion with fingertip blood sampling every 30 minutes for 2 hours. Results: Higher glucose levels in …
more
Transcriptional dysregulation of autophagy in the muscle of a mouse model of Duchenne muscular dystrophy
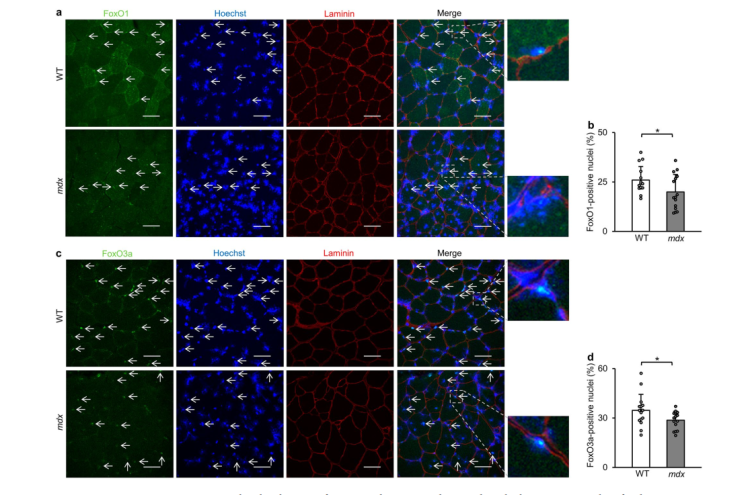
This study examines transcriptional dysregulation of autophagy in Duchenne muscular dystrophy, highlighting FoxO and TFEB suppression and resveratrol's therapeutic role.
more
Identifying hub genes and dysregulated pathways in Duchenne muscular dystrophy
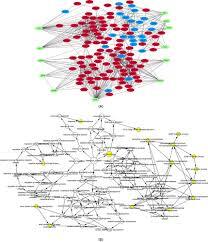
This research explores differentially expressed genes (DEGs) and hub genes in Duchenne muscular dystrophy, analyzing their role in disease progression and treatment targets.
more
Essential Terminology for Every Reader of Dystrophy Articles
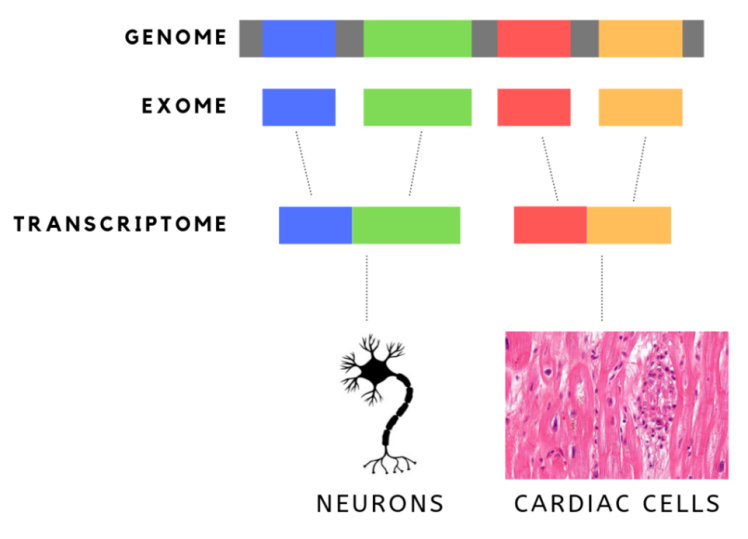
This article provides essential terminology for understanding muscular dystrophy research, covering key scientific terms such as cohort studies and exome sequencing.
more
Moving towards successful exon-skipping therapy for Duchenne muscular dystrophy

This review explores exon skipping therapy for Duchenne muscular dystrophy, discussing preclinical and clinical trials, therapeutic challenges, and future developments.
more
Exon skipping
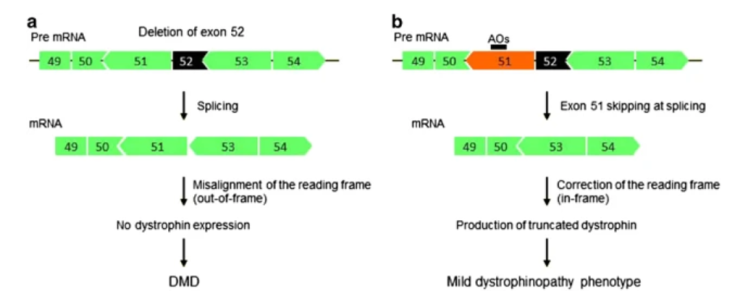
This article explains exon skipping, a genetic technique for treating Duchenne muscular dystrophy (DMD) by restoring the dystrophin protein using antisense oligonucleotides.
more
The Dysferlinopathies Conundrum: Clinical Spectra, Disease Mechanism and Genetic Approaches for Treatments

This review examines dysferlinopathies, highlighting clinical features, molecular mechanisms, genetic therapies, and the latest research in treatment development.
more
Duchenne muscular dystrophy: promising early-stage clinical trials to watch

This review highlights early-stage clinical trials for Duchenne muscular dystrophy, covering exon-skipping therapies, gene therapy, and novel treatment strategies.
more
Wnt7a is Required for Regeneration of Dystrophic Skeletal Muscle

This study examines the role of Wnt7a in muscle regeneration, highlighting its necessity for muscle repair and its potential as a therapy for Duchenne muscular dystrophy.
more
Isolation of small extracellular vesicles from regenerating muscle tissue using Tangential Flow Filt

This study presents an optimized protocol for isolating extracellular vesicles (EVs) from regenerating muscle tissue using TFF and SEC, ensuring purity and efficiency.
more
Satellite Cell Heterogeneity in Skeletal Muscle Homeostasis

This article explores the role of satellite cell heterogeneity in skeletal muscle homeostasis, highlighting its impact on muscle repair, self-renewal, and differentiation.
more
Several muscle diseases 5 : DYSF (Dysferlin) , Miyoshi Myopathy
This article explores DYSF mutations and Miyoshi Myopathy, detailing the role of dysferlin in muscle membrane repair and how its deficiency leads to muscle degeneration.
more
Several muscle diseases 4 : Skeletal Muscle FSHD

This article discusses facioscapulohumeral muscular dystrophy (FSHD), its genetic mechanisms, symptoms, and current research on potential treatments.
more
Several muscle diseases 3: LGMD2I, LGMD2B

This article discusses LGMD2I and LGMD2B, two subtypes of limb-girdle muscular dystrophy, their genetic causes, symptoms, and current management options.
more
Recent Posts
Categories
- DystroBlog | Neuromuscular Disease Research & News(96)
- Christopher W Ward(1)
- Jeffrey Chamberlain(2)
- Douglas Millay(1)
- Luis Garcia(1)
- Vincent Mouly(1)
- Bible(1)
- Yusuke Echigoya(2)
- Yuko Shimizu-Motohashi(1)
- Yu-Chiang Lai(1)
- Ype Elgersma(1)
- Yoshitsugu Aoki(1)
- Yetrib Hathout(1)
- Yasuhiro Yamada(1)
- W. David Arnold(1)
- Volker Straub(3)
- Virginia Arechavala-Gomeza(1)
- Vahab Soleimani(1)
- Utkarsh J. Dang(2)
- Toshifumi Yokota(13)
- Tony Kwan(1)
- Tobias Ruck(3)
- Tim Hagenacker(1)
- Thomas Sejersen(1)
- Theodore Perkins(1)
- Terry Partridge(1)
- Steve Wilton(1)
- Stephen Tapscott(1)
- Silvere van der Maarel(1)
- Shin’ichi Takeda(2)
- Ryszard Kole(1)
- Robert Weiss(1)
- Rob W.J. Collin(1)
- Rita Horvath(2)
- Renato Dulbecco(2)
- Rashmi Kothary(1)
- Rafal Czapiewski(1)
- Pim Pijnappel(1)
- Peter Meinke(1)
- Peter F M Van der Ven(2)
- Paula R. Clemens(2)
- Norio Motohashi(1)
- Muthiah Manoharan(1)
- Michela Guglieri(2)
- Michael W Lawlor(1)
- Michael A. Rudnicki(4)
- Már Tulinius(1)
- Masahisa Katsuno(1)
- Masad J. Damha(1)
- Linda Pax Lowes(1)
- Louise Rodino-Klapac(1)
- Leanne Ward(1)
- Larry Markham(1)
- Kristy Brown(1)
- Kevin Flanigan(2)
- Kate Bushby(1)
- Karim Wahbi(1)
- Karen Anthony(1)
- Kanneboyina Nagaraju(3)
- Julia M. Hum(1)
- Judith van Deutekom(1)
- Jose I. de las Heras(1)
- Joe Kornegay(1)
- Jin-Hong Shin(1)
- Jessica de Greef(1)
- Jerry R. Mendell(1)
- Jennifer Morgan(1)
- Jack H Vandermeulen(1)
- Hongping Zhang(1)
- Heike Kolbel(2)
- Hanns Lochmuller(2)
- Haluk Topaloglu(1)
- Guillaume Bourque(1)
- George Dickson(1)
- Gary Sweeney(1)
- Frank Rigo(1)
- Francesco Muntoni(2)
- Erik Landfeldt(2)
- Eric P. Hoffman(4)
- Eric C. Schirmer(1)
- En Kimura(1)
- Ehsan Javandoost(2)
- David L Mack(1)
- Davi Mazala(1)
- Craig M McDonald(1)
- Christian Werner(1)
- Capucine Trollet(1)
- Benedikt Schoser(1)
- Arnaud Ferry(1)
- Arezu Jahani-Asl(1)
- Antoni Borrell(1)
- Anthony Scimè(1)
- Annemieke Aartsma-Rus(5)
- Anne Schänzer(2)
- Anastasia Khvorova(1)
- Alyson Fiorillo(3)
- Allison D Ebert(1)
- Alessandra Sacco(1)
- Alastair R.W. Kerr(1)
- Akinori Nakamura(3)
- Terminology(10)
- Tools and Materials(5)
- Useful information(22)
- muscle diseases(6)
- Articles(72)
- DystroBlog | پژوهش و اخبار بیماریهای عصبی-عضلانی(96)
- Matthew S Alexander(1)
- Louis M. Kunkel(1)
- Karyn Esser(1)