more
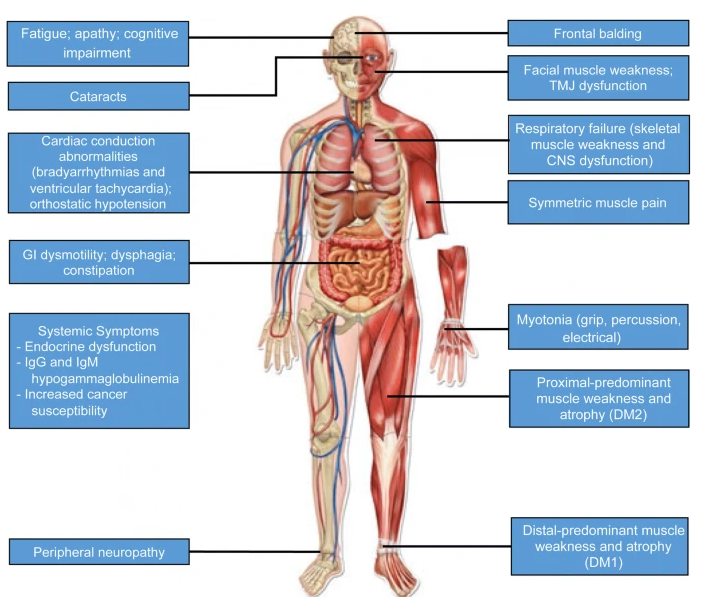
Golden Retriever Muscular Dystrophy (GRMD)
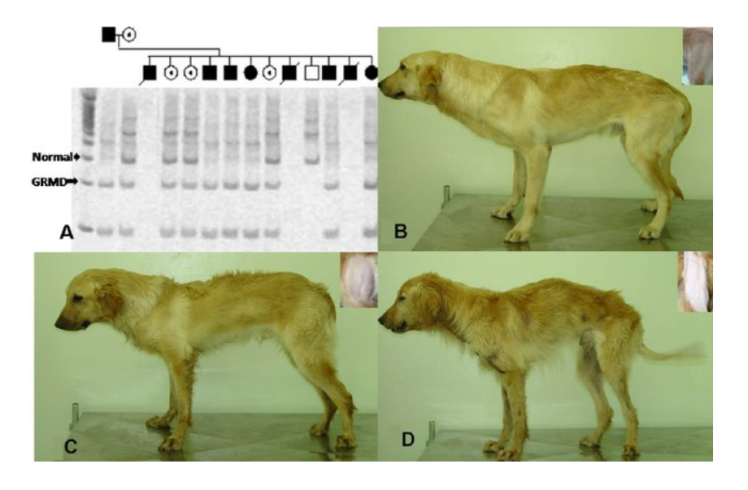
Golden Retriever Muscular Dystrophy (GRMD) is a genetic disorder in dogs that closely resembles Duchenne muscular dystrophy (DMD) in humans. It serves as a valuable model for studying therapies, including gene therapy and pharmacological treatments.
more
Predictors of cardiac disease in duchenne muscular dystrophy: a systematic review and evidence grading

This systematic review evaluates predictors of cardiac disease in Duchenne muscular dystrophy, grading evidence on pharmacological treatments, genetic modifiers, and interventions.
more
Joining forces to develop individualized antisense oligonucleotides for patients with brain or eye diseases: the example of the Dutch Center for RNA Therapeutics

This article explores how the Dutch Center for RNA Therapeutics (DCRT) is advancing individualized antisense oligonucleotide (ASO) therapies for rare brain and eye diseases.
more
Thrombospondin-4 as potential cerebrospinal fluid biomarker for therapy response in pediatric spinal muscular atrophy

This study explores Thrombospondin-4 as a cerebrospinal fluid biomarker for therapy response in pediatric SMA patients, highlighting its potential for treatment monitoring.
more
Diagnosis of Duchenne Muscular Dystrophy in a Presymptomatic Infant Using Next-Generation Sequencing and Chromosomal Microarray Analysis: A Case Report

This case report highlights the early diagnosis of Duchenne muscular dystrophy (DMD) in a presymptomatic infant using NGS and CMA, emphasizing the benefits of genetic testing.
more
Effectiveness of Nusinersen in Adolescents and Adults with Spinal Muscular Atrophy: Systematic Review and Meta‑analysis

This study evaluates the effectiveness of Nusinersen in adolescents and adults with SMA, highlighting improvements in motor function based on HFMSE, RULM, and 6MWT scores.
more
Chemistry, structure and function of approved oligonucleotide therapeutics

This article reviews the chemistry, structure, and function of oligonucleotide therapeutics, highlighting their modifications, delivery methods, and clinical successes.
more
Ubiquitylomics: An Emerging Approach for Profiling Protein Ubiquitylation in Skeletal Muscle

This article reviews ubiquitylomics, a novel approach for studying protein ubiquitylation in skeletal muscle, highlighting its role in muscle health, disease, and therapeutics.
more
Current Outline of Exon Skipping Trials in Duchenne Muscular Dystrophy

This article reviews current exon skipping trials for Duchenne muscular dystrophy, focusing on FDA-approved AON therapies, challenges, and future research directions.
more
Knockdown of CDR1as Decreases Differentiation of Goat Skeletal Muscle Satellite Cells via Upregulating miR-27a-3p to Inhibit ANGPT1

This study reveals that CDR1as knockdown in goat skeletal muscle satellite cells increases miR-27a-3p expression, leading to ANGPT1 inhibition and reduced differentiation.
more
Antisense and Gene Therapy Options for Duchenne Muscular Dystrophy Arising from Mutations in the N-Terminal Hotspot

This article reviews antisense and gene therapy strategies for DMD mutations in the exon 2–22 region, focusing on exon skipping, AAV vectors, and microdystrophin therapy.
more
Nucleic Acid-Based Therapeutic Approach for Spinal and Bulbar Muscular Atrophy and Related Neurological Disorders

This review discusses nucleic acid-based therapies for SBMA, including ASOs, siRNAs, and gene therapy, focusing on their potential to slow disease progression and improve muscle function.
more
Consensus Guidelines for the Design and In Vitro Preclinical Efficacy Testing N-of-1 Exon Skipping Antisense Oligonucleotides

This article outlines standardized guidelines for developing N-of-1 exon skipping antisense oligonucleotides, emphasizing preclinical testing, ASO design, and data sharing.
more
Guidelines for Antisense Oligonucleotide Design and Insight Into Splice-modulating Mechanisms

This study presents optimized guidelines for designing antisense oligonucleotides, analyzing their sequence composition, binding energy, and role in exon skipping therapies.
more
Recent Posts
Categories
- DystroBlog | Neuromuscular Disease Research & News(96)
- Christopher W Ward(1)
- Jeffrey Chamberlain(2)
- Douglas Millay(1)
- Luis Garcia(1)
- Vincent Mouly(1)
- Bible(1)
- Yusuke Echigoya(2)
- Yuko Shimizu-Motohashi(1)
- Yu-Chiang Lai(1)
- Ype Elgersma(1)
- Yoshitsugu Aoki(1)
- Yetrib Hathout(1)
- Yasuhiro Yamada(1)
- W. David Arnold(1)
- Volker Straub(3)
- Virginia Arechavala-Gomeza(1)
- Vahab Soleimani(1)
- Utkarsh J. Dang(2)
- Toshifumi Yokota(13)
- Tony Kwan(1)
- Tobias Ruck(3)
- Tim Hagenacker(1)
- Thomas Sejersen(1)
- Theodore Perkins(1)
- Terry Partridge(1)
- Steve Wilton(1)
- Stephen Tapscott(1)
- Silvere van der Maarel(1)
- Shin’ichi Takeda(2)
- Ryszard Kole(1)
- Robert Weiss(1)
- Rob W.J. Collin(1)
- Rita Horvath(2)
- Renato Dulbecco(2)
- Rashmi Kothary(1)
- Rafal Czapiewski(1)
- Pim Pijnappel(1)
- Peter Meinke(1)
- Peter F M Van der Ven(2)
- Paula R. Clemens(2)
- Norio Motohashi(1)
- Muthiah Manoharan(1)
- Michela Guglieri(2)
- Michael W Lawlor(1)
- Michael A. Rudnicki(4)
- Már Tulinius(1)
- Masahisa Katsuno(1)
- Masad J. Damha(1)
- Linda Pax Lowes(1)
- Louise Rodino-Klapac(1)
- Leanne Ward(1)
- Larry Markham(1)
- Kristy Brown(1)
- Kevin Flanigan(2)
- Kate Bushby(1)
- Karim Wahbi(1)
- Karen Anthony(1)
- Kanneboyina Nagaraju(3)
- Julia M. Hum(1)
- Judith van Deutekom(1)
- Jose I. de las Heras(1)
- Joe Kornegay(1)
- Jin-Hong Shin(1)
- Jessica de Greef(1)
- Jerry R. Mendell(1)
- Jennifer Morgan(1)
- Jack H Vandermeulen(1)
- Hongping Zhang(1)
- Heike Kolbel(2)
- Hanns Lochmuller(2)
- Haluk Topaloglu(1)
- Guillaume Bourque(1)
- George Dickson(1)
- Gary Sweeney(1)
- Frank Rigo(1)
- Francesco Muntoni(2)
- Erik Landfeldt(2)
- Eric P. Hoffman(4)
- Eric C. Schirmer(1)
- En Kimura(1)
- Ehsan Javandoost(2)
- David L Mack(1)
- Davi Mazala(1)
- Craig M McDonald(1)
- Christian Werner(1)
- Capucine Trollet(1)
- Benedikt Schoser(1)
- Arnaud Ferry(1)
- Arezu Jahani-Asl(1)
- Antoni Borrell(1)
- Anthony Scimè(1)
- Annemieke Aartsma-Rus(5)
- Anne Schänzer(2)
- Anastasia Khvorova(1)
- Alyson Fiorillo(3)
- Allison D Ebert(1)
- Alessandra Sacco(1)
- Alastair R.W. Kerr(1)
- Akinori Nakamura(3)
- Terminology(10)
- Tools and Materials(5)
- Useful information(22)
- muscle diseases(6)
- Articles(72)
- DystroBlog | پژوهش و اخبار بیماریهای عصبی-عضلانی(96)
- Matthew S Alexander(1)
- Louis M. Kunkel(1)
- Karyn Esser(1)